Abstract
The title compound 6-(4-amino-1-methyl-2-(methylthio)-6-oxo-1,6-dihydro-pyrimidin-5-yl)-3,6-dimethyl-2-(methylthio)-6,7-dihydro-3H-pyrrolo[2,3-d]pyrimidine-4,5-dione was synthesized in 60% yield by a microwave-induced cyclocondensation reaction of aminopyrimidine with pyruvic acid in the presence of cerium ammonium nitrate (CAN) as catalyst.
Introduction
Nitrogen heterocycles have received a great deal of attention in the literature as a result of their role as pharmacophores of great historical significance. Among these heterocyclic systems, those containing pyrimidine in particular have been the subject of expanding research efforts in heteroaromatic and biological chemistry.
The structural diversity and biological importance of pyrimidines have made them attractive synthesis targets for many years. The pyrimidine is a widespread heterocyclic moiety, present in numerous natural products as well as synthetic pharmacophores with biological activities [1,2,3,4]. Substituted pyrimidines, particularly with amino groups at the 2 and 4 positions, are known pharmacophores in several structure-based drug design approaches in medicinal chemistry [5,6,7]. Pyrimidines and their fused derivatives have been studied continuously because they exhibit broad biological activity as antitumor [8,9,10,11], antifungal [12,13], antibacterial [12,14,15,16], anti-HIV agents [17,18,19]. The synthesis of pyrrolopyrimidines is of high interest in medicinal chemistry, because some of them possess biological and pharmacological activities, such as anti-leukemia [20], tyrosine kinase inhibitors [20,21,22,23], anti-HIV-1 [24], antibiotic [25], antiangiogenic and antitumor properties [20]. Syntheses of pyrrolopyrimidines have been reported by several authors. Generally an aminopyrimidine reacts with either an α-halo-aldehyde [26,27], α-halo-ketone [28,29] or α-halo-acid chloride [29].
In continuation of our previous studies of the synthesis of heterocyclic compounds from heterocyclic amines [30,31,32,33,34,35,36], in this work a novel pyrrolo[2,3-d]pyrimidine synthesis was performed, where the target compound was obtained by the reaction between the aminopyrimidine and pyruvic acid (an α-keto-acid) using a microwave irradiation and cerium ammonium nitrate (CAN) as catalyst.
Results and Discussion
The synthesis of 6-(4-amino-1-methyl-2-(methylthio)-6-oxo-1,6-dihydropyrimidin-5-yl)-3,6-dimethyl-2-(methylthio)-6,7-dihydro-3H-pyrrolo[2,3-d]pyrimidine-4,5-dione involves the reaction of aminopyrimidine 1 (2 eq.) with pyruvic acid (2, 1 eq.) in ethanol (Scheme 1). The reaction mixture was irradiated with microwaves at 80 °C for 8 minutes; cerium ammonium nitrate (CAN) was used as the catalyst. The reaction was monitored using thin layer chromatography. The yellow cream solid formed was filtered under reduced pressure and did not require further purification.
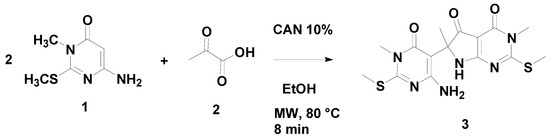
Scheme 1.
Synthesis of 6-(4-amino-1-methyl-2-(methylthio)-6-oxo-1,6-dihydropyrimidin-5-yl)-3,6-dimethyl-2-(methylthio)-6,7-dihydro-3H-pyrrolo[2,3-d]pyrimidine-4,5-dione.
The structure of the compound 3 was determined by spectroscopic techniques and mass spectrometry. The proton NMR spectrum showed signals for nonequivalent SCH3 and NCH3 groups at 2.54, 2.60, 3.20 and 3.39 ppm as a singlets, respectively, whereas a singlet at δ 1.55 ppm was assigned to the 6-CH3 protons and two singlets at 6.45 and 10.83 ppm correspond to the NH2 group and NH of the pyrrole ring. The IR spectrum analysis showed the NH band at 3402 and 3329 cm−1, and carbonyl bands at 1751 and 1632 cm−1.
A plausible mechanism is shown in Scheme 2. Initially the CAN, dissociates into its constituents. It is known that the nitrate anion and the ammonium cation can react to form nitric acid and ammonia. The Ce(IV) is coordinated with the carbonyl groups [37] of pyruvic acid, and the C-5 carbon of the aminopyrimidine performs a nucleophilic attack on the carbonyl group of the carboxylic acid to form intermediate Ia, which loses water and forms Ib. Compound Ib subsequently reacts with another mole of aminopyrimidine to form IIa-b, and the acidic medium promotes the decoupling of the Ce(IV) from the carbonyl groups and allows a second dehydration, followed by the cyclization of the second amino group to form the desired pyrrolo[2,3-d]pyrimidine 3.
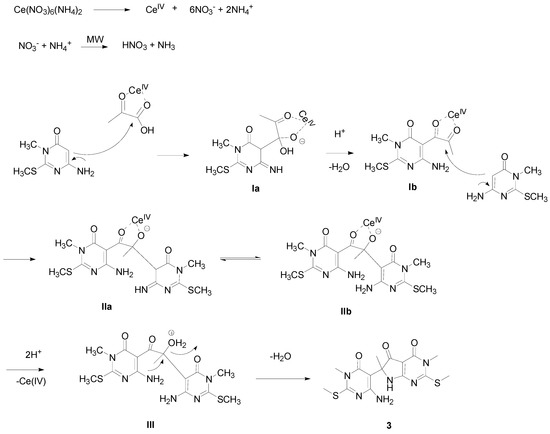
Scheme 2.
Plausible mechanism for the formation of the new pyrrolo[2,3-d]pyrimidine.
Experimental
General Information
The reaction progress was monitored by precoated TLC plates of silica gel 60GF254 of 0.2 µm thickness (Merck, Darmstadt, Germany). Melting points were measured using a Stuart SMP3 melting point apparatus and are uncorrected. IR spectra were obtained with an IR Affinity-1 instrument (Shimadzu, Kyoto, Japan) equipped with an ATR accessory. The 1H and 13C-NMR spectra were run on a DPX 400 spectrometer (Bruker, Bruker BioSpin GmbH, Rheinstetten, Germany) operating at 400 and 101 MHz respectively, using dimethyl sulfoxide-d6 as solvent and TMS as internal standard. The mass spectrum was obtained on a Shimadzu-GCMS-QP2010 spectrometer operating at 70 eV. Microwave experiments were carried out in a CEM Discover SystemTM 300 W focused microwave reactor (manufacturer, Charlotte, NC, USA).
Procedure for the Synthesis of 6-(4-Amino-1-methyl-2-(methylthio)-6-oxo-1,6-dihydropyrimidin-5-yl)-3,6-dimethyl-2-(methylthio)-6,7-dihydro-3H-pyrrolo[2,3-d]pyrimidine-4,5-dione
A mixture of 6-aminopyrimidine 1 (2 mmol) and pyruvic acid (2, 1 mmol) and CAN 10% mol in ethanol (1 mL) was heated by microwave irradiation for 8 minutes (80 °C). The solid was filtered under reduced pressure and washed with ethanol. Compound 3 was obtained in high purity (according to TLC and the corresponding NMR spectrum) and did not require further recrystallization. Yellow cream solid, Yield: 60% M.p.: 254 °C (dec). IR (ATR) (cm−1): 3402 (NH), 3329 (NH), 3217 (C-H), 1751 (C=O), 1632 (C=O). 1H-NMR (DMSO-d6) δ ppm: 1.55 (s, 3H, CH3), 2.54 (s, 3H, SCH3), 2.60 (s, 3H, SCH3), 3.20 (s, 3H, N-CH3), 3.39 (s, 3H, N-CH3), 6.45 (s, 2H, NH2), 10.83 (s, 1H, NH). 13C-NMR (DMSO-d6) δ ppm: 14.5 (SCH3), 15.2 (SCH3), 20.2 (CH3), 29.9 (N-CH3), 30.2 (N-CH3), 47.2 (C), 94.4 (C), 101.6 (C), 157.9 (C), 158.9 (C), 159.9 (C), 160.9 (C), 165.0 (C), 181.9 (C). MS (EI): m/z 394 (57, [M+]), 379 (77, M+ - CH3). Anal. Calcd. For C15H18N6O3S2 (394.47): C: 45.67; H: 4.60; N: 21.31; Found: C: 45.51; H: 4.49; N: 21.44.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
Authors wish to thank the COLCIENCIAS and Universidad del Valle for financial support.
Author Contributions
The authors PER, JQ, BI, MN and JC designed and accomplished research. Also, they analyzed data and wrote the paper together. Finally, all authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest
References
- Choudhury, A.; Chen, H.; Nilsen, C.N.; Sorgi, K.L. A chemoselective aniline–chloropyrimidine coupling in a competing electrophilic environment. Tetrahedron Lett. 2008, 49, 102–105. [Google Scholar] [CrossRef]
- Brændvang, M.; Gundersen, L.-L. Efficient and regioselective N-1 alkylation of 4-chloropyrazolo[3,4-d]pyrimidine. Tetrahedron Lett. 2007, 48, 3057–3059. [Google Scholar] [CrossRef]
- Peng, Z.; Journet, M.; Humphrey, G. A Highly Regioselective Amination of 6-Aryl-2,4-dichloropyrimidine. Org. Lett. 2006, 8, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Girreser, U.; Heber, D.; Schütt, M. Synthesis of 6-substituted 7-aryl-5,6-dihydropyrido[2,3-d]pyrimidine(1H,3H)-2,4-diones using the Vilsmeier reaction. Tetrahedron 2004, 60, 11511–11517. [Google Scholar] [CrossRef]
- Boudet, N.; Knochel, P. Chemo- and regioselective functionalization of uracil derivatives. Applications to the synthesis of oxypurinol and emivirine. Org. Lett. 2006, 8, 3737–40. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.; Bovero, A.; Fruttarolo, F.; Romagnoli, R.; Aghzadeh, M.; Preti, D.; Varani, K.; Borea, P.; Moorman, A. New strategies for the synthesis of A3 adenosine receptor antagonists. Bioorg. Med. Chem. 2003, 11, 4161–4169. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Cacciari, B.; Romagnoli, R.; Spalluto, G.; Moro, S.; Klotz, K.; Leung, E.; Varani, K.; Gessi, S.; Merighi, S.; et al. Selective Human A3 Adenosine Receptor Antagonists: Influence of the Chain at the N8 Pyrazole Nitrogen. J. Med. Chem. 2000, 43, 4768–4780. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.S.; El-Bendary, E.R.; El-Ashry, S.M.; El-Kerdawy, M.M. Synthesis and antitumor activity of new sulfonamide derivatives of thiadiazolo[3,2-a]pyrimidines. Eur. J. Med. Chem. 2011, 46, 3714–3720. [Google Scholar] [CrossRef] [PubMed]
- Fares, M.; Abou-Seri, S.M.; Abdel-Aziz, H.; Abbas, S.; Youssef, M.M.; Eladwy, R.A. Synthesis and antitumor activity of pyrido [2,3-d]pyrimidine and pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidine derivatives that induce apoptosis through G1 cell-cycle arrest. Eur. J. Med. Chem. 2014, 83, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Al-Omary, F.A.M.; Hassan, G.S.; El-Messery, S.M.; El-Subbagh, H.I. Substituted thiazoles V. synthesis and antitumor activity of novel thiazolo[2,3-b]quinazoline and pyrido[4,3-d]thiazolo[3,2-a]pyrimidine analogues. Eur. J. Med. Chem. 2012, 47, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, S.; Wang, Y.; Li, R.; Wang, J.; Wang, L.; Zhao, Y.; Gong, P. Design, synthesis and biological evaluation of novel thieno[3,2-d]pyrimidine derivatives possessing diaryl semicarbazone scaffolds as potent antitumor agents. Eur. J. Med. Chem. 2014, 87, 782–93. [Google Scholar] [CrossRef] [PubMed]
- Hilmy, K.M.H.; Khalifa, M.M.A.; Hawata, M.A.A.; Keshk, R.M.A.; El-Torgman, A.A. Synthesis of new pyrrolo[2,3-d]pyrimidine derivatives as antibacterial and antifungal agents. Eur. J. Med. Chem. 2010, 45, 5243–5250. [Google Scholar] [CrossRef] [PubMed]
- Gholap, A.R.; Toti, K.S.; Shirazi, F.; Deshpande, M.V.; Srinivasan, K.V. Efficient synthesis of antifungal pyrimidines via palladium catalyzed Suzuki/Sonogashira cross-coupling reaction from Biginelli 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron 2008, 64, 10214–10223. [Google Scholar] [CrossRef]
- Bhalgat, C.M.; Ramesh, B. Synthesis, antimicrobial screening and structure–activity relationship of novel pyrimidines and their thioethers. Bull. Fac. Pharmacy, Cairo Univ. 2014, 52, 259–267. [Google Scholar] [CrossRef]
- Saikia, L.; Das, B.; Bharali, P.; Thakur, A.J. A convenient synthesis of novel 5-aryl-pyrido[2,3-d]pyrimidines and screening of their preliminary antibacterial properties. Tetrahedron Lett. 2014, 55, 1796–1801. [Google Scholar] [CrossRef]
- Al-Adiwish, W.M.; Tahir, M.I.M.; Siti-Noor-Adnalizawati, A.; Hashim, S.F.; Ibrahim, N.; Yaacob, W. Synthesis, antibacterial activity and cytotoxicity of new fused pyrazolo[1,5-a]pyrimidine and pyrazolo[5,1-c][1,2,4]triazine derivatives from new 5-aminopyrazoles. Eur. J. Med. Chem. 2013, 64, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Wallis, M.P.; Mahmood, N.; Fraser, W. Synthesis and anti-HIV activity of C4-modified pyrimidine nucleosides. Il Farmaco 1999, 54, 83–89. [Google Scholar] [CrossRef]
- Gazivoda, T.; Raić-Malić, S.; Kristafor, V.; Makuc, D.; Plavec, J.; Bratulić, S.; Kraljević-Pavelić, S.; Pavelić, K.; Naesens, L.; Andrei, G.; et al. Synthesis, cytostatic and anti-HIV evaluations of the new unsaturated acyclic C-5 pyrimidine nucleoside analogues. Bioorg. Med. Chem. 2008, 16, 5624–5634. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Du, D.; Rai, D.; Wang, L.; Liu, H.; Zhan, P.; de Clercq, E.; Pannecouque, C.; Liu, X. Fused heterocyclic compounds bearing bridgehead nitrogen as potent HIV-1 NNRTIs. Part 1: design, synthesis and biological evaluation of novel 5,7-disubstituted pyrazolo[1,5-a]pyrimidine derivatives. Bioorg. Med. Chem. 2014, 22, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Gangjee, A.; Namjoshi, O.; Yu, J.; Ihnat, M.; Thorpe, J.E.; Bailey-Downs, L.C. N2-Trimethylacetyl substituted and unsubstituted-N4-phenylsubstituted-6-(2-pyridin-2-ylethyl)-7H-pyrrolo[2,3-d]pyrimidine-2,4-diamines: Design, cellular receptor tyrosine kinase inhibitory activities and in vivo evaluation as antiangiogenic, antimetastatic and antitumor agents. Bioorg. Med. Chem. 2013, 21, 1312–1323. [Google Scholar] [PubMed]
- Gangjee, A.; Zaware, N.; Raghavan, S.; Yang, J.; Thorpe, J.E.; Ihnat, M.A. N4-(3-Bromophenyl)-7-(substituted benzyl) pyrrolo[2,3-d]pyrimidines as potent multiple receptor tyrosine kinase inhibitors: Design, synthesis, and in vivo evaluation. Bioorg. Med. Chem. 2012, 20, 2444–2454. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.D.; Wilson, J.W.; Deanda, F.; Patnaik, S.; Redman, A.M.; Yang, B.; Shewchuk, L.; Sabbatini, P.; Leesnitzer, M.A.; Groy, A.; et al. Discovery of 4,6-bis-anilino-1H-pyrrolo[2,3-d]pyrimidines: Potent inhibitors of the IGF-1R receptor tyrosine kinase. Bioorg. Med. Chem. Lett. 2009, 19, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Kaspersen, S.J.; Sørum, C.; Willassen, V.; Fuglseth, E.; Kjøbli, E.; Bjørkøy, G.; Sundby, E.; Hoff, B.H. Synthesis and in vitro EGFR (ErbB1) tyrosine kinase inhibitory activity of 4-N-substituted 6-aryl-7H-pyrrolo[2,3-d]pyrimidine-4-amines. Eur. J. Med. Chem. 2011, 46, 6002–6014. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Y.; Tao, L.; Wang, Q.; Wang, S.; Hu, W.; Pan, Z.; Yang, Q.; Cui, Y.; Ge, Z.; et al. Synthesis and anti-HIV-1 activity of 4-substituted-7-(2'-deoxy-2'-fluoro-4'-azido-β-d-ribofuranosyl)pyrrolo[2,3-d]pyrimidine analogues. Bioorg. Med. Chem. Lett. 2011, 21, 6770–6772. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.P.; Frey, K.M.; Wang, Y.; Jain, H.K.; Gangjee, A.; Anderson, K.S. Substituted pyrrolo[2,3-d]pyrimidines as Cryptosporidium hominis thymidylate synthase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 5426–5428. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, J.; Acosta, P.A.; Cruz, S.; Abonía, R.; Insuasty, B.; Nogueras, M.; Cobo, J. Generation of pyrrolo[2,3-d]pyrimidines. Unexpected products in the multicomponent reaction of 6-aminopyrimidines, dimedone, and arylglyoxal. Tetrahedron Lett. 2010, 51, 5443–5447. [Google Scholar] [CrossRef]
- Barnett, C.J.; Grubb, L.M. Synthesis of pyrrolo[2,3-d]pyrimidines via cyclocondensation of β-alkoxy and β-amino-α-bromoaldehydes. Tetrahedron Lett. 2000, 41, 9741–9745. [Google Scholar] [CrossRef]
- Bundy, G.L.; Ayer, D.E.; Banitt, L.S.; Belonga, K.L.; Mizsak, S.A.; Palmer, J.R.; Tustin, J.M.; Chin, J.E.; Hall, E.D.; Linseman, K.L.; et al. Synthesis of Novel 2,4-Diaminopyrrolo-[2,3-d]pyrimidines with Antioxidant, Neuroprotective, and Antiasthma Activity. J. Med. Chem. 1995, 38, 4161–4163. [Google Scholar] [CrossRef] [PubMed]
- Lipton, M.F.; Mauragis, M.A.; Veley, M.F.; Bundy, G.L.; Banitt, L.S.; Dobrowolski, P.J.; Palmer, J.R.; Schwartz, T.M.; Zimmerman, D.C. Four Generations of Pyrrolopyrimidines. In From Bench to Pilot Plant; Nafissi, M., Ragan, J.A., DeVries, K.M., Eds.; American Chemical Society: Washington, DC, USA, 2002; pp. 101–112. [Google Scholar]
- Gálvez, J.; Quiroga, J.; Insuasty, B.; Abonia, R. Microwave-assisted and iodine mediated synthesis of 5-n-alkyl-cycloalkane[d]-pyrazolo[3,4-b]pyridines from 5-aminopyrazoles and cyclic ketones. Tetrahedron Lett. 2014, 55, 1998–2002. [Google Scholar] [CrossRef]
- Quiroga, J.; Diaz, Y.; Bueno, J.; Insuasty, B.; Abonia, R.; Ortiz, A.; Nogueras, M.; Cobo, J. Microwave induced three-component synthesis and antimycobacterial activity of benzopyrazolo[3,4-b]quinolindiones. Eur. J. Med. Chem. 2014, 74, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Insuasty, B.; Becerra, D.; Quiroga, J.; Abonia, R.; Nogueras, M.; Cobo, J. Synthesis of Novel Pyrimido[4,5-b]quinolin-4-ones with Potential Antitumor Activity. J. Heterocycl. Chem. 2013, 50, 506–512. [Google Scholar] [CrossRef]
- Insuasty, B.; Becerra, D.; Quiroga, J.; Abonia, R.; Nogueras, M.; Cobo, J. Microwave-assisted synthesis of pyrimido[4,5-b][1,6]naphthyridin-4(3H)-ones with potential antitumor activity. Eur. J. Med. Chem. 2013, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, J.; Portillo, S.; Pérez, A.; Gálvez, J.; Abonia, R.; Insuasty, B. An efficient synthesis of pyrazolo[3,4-b]pyridine-4-spiroindolinones by a three-component reaction of 5-aminopyrazoles, isatin, and cyclic β-diketones. Tetrahedron Lett. 2011, 52, 2664–2666. [Google Scholar] [CrossRef]
- Quiroga, J.; Trilleras, J.; Pantoja, D.; Abonía, R.; Insuasty, B.; Nogueras, M.; Cobo, J. Microwave-assisted synthesis of pyrazolo[3,4-b]pyridine-spirocycloalkanediones by three-component reaction of 5-aminopyrazole derivatives, paraformaldehyde and cyclic β-diketones. Tetrahedron Lett. 2010, 51, 4717–4719. [Google Scholar] [CrossRef]
- Quiroga, J.; Trilleras, J.; Insuasty, B.; Abonía, R.; Nogueras, M.; Marchal, A.; Cobo, J. A straightforward synthesis of pyrimido[4,5-b]quinoline derivatives assisted by microwave irradiation. Tetrahedron Lett. 2010, 51, 1107–1109. [Google Scholar] [CrossRef]
- Sridharan, V.; Mene, J.C. Cerium (IV) Ammonium Nitrate as a Catalyst in Organic Synthesis. Chem. Rev. 2010, 110, 3805–3849. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).