Abstract
4-(5-(2-Methoxyphenyl)-1,3,4-oxadiazol-2-yl)benzohydrazide (5) was synthesized by three steps. The synthesis started with 2-methoxybenzohydrazide to form hydrazone (3) which was then cyclized to oxadiazole (4) and finally, treatment of oxadiazole (4) with hydrazine hydrate afforded the final product (5).
Introduction
Benzohydrazides have been reported to possess various biological activities such as antileishmanial [1], antioxidant [2] or antibacterial effects [3]. Benzohydrazides are easily converted into hydrazones by treating with aldehyde or ketone [4]. Benzohydrazones are applicable in mass spectrometry as alternate UV-LDI matrices for the analysis of peptides with significantly low background signals [5]. Recent studies on benzohydrazones also showed that they are potent antileishmanial [6] antioxidant [7,8] antidiabetic [9,10] antibacterial [3] and antifungal [11] agents. Benzohydrazones can be converted into oxadiazole by using cerium ammonium nitrate (CAN) [12], bis(trifluoroacetoxy)iodobenzene [13] or Dess–Martin reagent (DMP) [14]. Literature search showed that oxadiazole carrying a benzoyl hydrazine moiety has not been reported. Therefore, 4-(5-(2-methoxyphenyl)-1,3,4-oxadiazol-2-yl)benzohydrazide (5), a new oxadiazole containing a benzoyl hydrazine unit, was obtained as shown in Scheme 1. The product was prepared with satisfactory yield and fully characterized by spectroscopy.
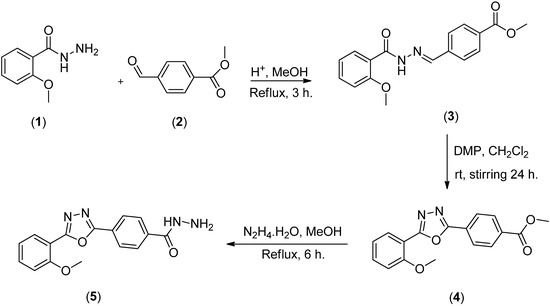
Scheme 1.
Synthesis of 4-(5-(2-methoxyphenyl)-1,3,4-oxadiazol-2-yl)benzohydrazide.
Results and Discussion
We have synthesized (E)-methyl 4-((2-(2-methoxybenzoyl)hydrazinylidene)methyl)benzoate (3) by condensing 2-methoxybenzohydrazide (1) with methyl 4-formylbenzoate (2) [6]. Compound 3 was then cyclized to form methyl 4-(5-(2-methoxyphenyl)-1,3,4-oxadiazol-2-yl)benzoate (4) by using DMP [13]. Finally oxadiazole 4 was refluxed with hydrazine hydrate for 6 hours to give 4-(5-(2-methoxyphenyl)-1,3,4-oxadiazol-2-yl)benzohydrazide (5) in high yield (Scheme 1).
Experimental
4-(5-(2-Methoxyphenyl)-1,3,4-oxadiazol-2-yl)benzohydrazide
4-(5-(2-Methoxyphenyl)-1,3,4-oxadiazol-2-yl)benzohydrazide (5) was synthesized by mixing compound 4 (0.620 g, 2 mmol) with 2 mL hydrazine hydrate and 10 mL methanol. The mixture was refluxed for 6 h. Excess hydrazine hydrate and solvent were evaporated to afford pure product 5 (94%, yield 0.58 g). M.p. above 250 °C; 1H-NMR (500 MHz, DMSO-d6): 10.01 (s, 1H, NH), δ 8.14 (d, 2H, J2’,3’ = J6’,5’ = 8.5 Hz, H-2’/H-6’), 8.02 (d, 2H, J3’,2’ = J5’,6’ = 8.5 Hz, H-3’/H-5’), 7.97 (dd, 1H, J3,4 = 6.0, J3,5 = 2.0 Hz, H-3), 7.64 (ddd, 1H, J4,3 = 7.5, J4,5 = 6.5, J4,6 = 2.0 Hz, H-4), 7.29 (d, 1H, J6,5 = 8.5, H-6), 7.16 (t, 1H, J5(6,4) = 8.5, H-5), 4.58 (s, 2H, NH2), 3.93 (s, 3H, O-CH3); 13C-NMR (125 MHz, DMSO-d6): δ 165.41, 163.79, 163.59, 158.02, 136.50, 134.22, 130.70, 130.70, 128.48, 128.48, 127.05, 125.98, 121.28, 113.22, 112.45, 56.55; Anal. Calcd for C16H14N4O3, C, 61.93; H, 4.55; N, 18.06; Found C, 61.95; H, 4.56; N, 18.04; EI MS m/z 310.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Authors would like to acknowledge Research Management Institute of UiTM for the financial support under CIFI Scheme 600-RMI/DANA 5/3/CIFI (141/2013).
Author Contributions
Muhammad Taha designed the experiment and synthesized the compound. Syahrul Imran analyzed the compound using NMR. Nor Hadiani Ismail and Khalid Muhammad Khan write the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Alptuzun, V.; Cakiroglu, G.; Limoncu, M.E.; Erac, B.; Hosgor-Limoncu, M.; Erciyas, E. Synthesis and antileishmanial activity of novel pyridinium-hydrazone derivatives. J. Enzyme Inhib. Med. Chem. 2013, 28, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Li, T.R.; Yang, Z.Y.; Wang, B.D. Synthesis, characterization and antioxidant activity of naringenin Schiff base and its Cu(II), Ni(II), Zn(II) complexes. Chem. Pharm. Bull. 2007, 55, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Khattab, S.N. Synthesis and Biological Activity of Novel Amino Acid-(N'-Benzoyl) Hydrazide and Amino Acid-(N'-Nicotinoyl) Hydrazide Derivatives. Molecules 2005, 10, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Jamil, W.; Ambreen, N.; Taha, M.; Perveen, S.; Morales, G.A. An Expeditious Synthetic Approach towards the Synthesis of Bis-Schiff Bases (Aldazines) Using Ultrasound. Ultrason. Sonochem. 2014, 21, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Musharraf, S.G.; Bibi, A.; Shahid, N.; Najam-ul-Haq, M.; Khan, M.; Taha, M.; Mughal, U.R.; Khan, K.M. Acylhydrazide and Isatin Schiff Bases as Alternate UV-Laser Desorption Ionization (LDI) Matrices for Low Molecular Weight (LMW) Peptides Analysis. Am. J. Anal. Chem. 2012, 3, 779–789. [Google Scholar] [CrossRef]
- Taha, M.; Baharudin, M.S.; Ismail, N.H.; Khan, K.M.; Jaafar, F.M.; Samreen; Siddiqui, S.; Choudhary, M.I. Synthesis of 2-methoxybenzoylhydrazone and evaluation of their antileishmanial activity. Bioorg. Med. Chem. Lett. 2013, 23, 3463–3466. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Ismail, N.H.; Jamil, W.; Yousuf, S.; Jaafar, F.M.; Ali, M.I.; Kashif, S.M.; Hussain, E. Synthesis, Evaluation of Antioxidant Activity and Crystal Structure of 2,4-Dimethylbenzoylhydrazones. Molecules 2013, 18, 10912–10929. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Shah, Z.; Ahmad, V.U.; Khan, M.; Taha, M.; Ali, S.; Perveen, S.; Choudhary, M.I.; Voelter, W. 2,4,6-Trichlorophenylhydrazine Schiff Bases as DPPH Radical and Super Oxide Anion Scavengers. Med. Chem. 2012, 8, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Naz, H.; Rasheed, S.; Ismail, N.H.; Rahman, A.A.; Yousuf, S.; Choudhary, M.I. Synthesis of 4-Methoxybenzoylhydrazones and Evaluation of Their Antiglycation Activity. Molecules 2014, 19, 1286–1301. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Taha, M.; Rahim, F.; Fakhri, M.I.; Jamil, W.; Khan, M.; Rasheed, S.; Karim, A.; Perveen, S.; Choudhary, M.I. Acylhydrazide Schiff Bases: Synthesis and Antiglycation Activity. J. Chem. Soc. Pak. 2013, 35, 929–937. [Google Scholar]
- Sundriyal, S.; Sharma, R.K.; Jain, R. Current advances in antifungal targets and drug development. Curr. Med. Chem. 2006, 13, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, M.; Salehi, P.; Baghbanzadeha, M.; Bahramnejada, M. A facile procedure for the one-pot synthesis of unsymmetrical 2,5-disubstituted 1,3,4-oxadiazoles. Tetrahedron Lett. 2006, 47, 6983–6986. [Google Scholar] [CrossRef]
- Shang, Z.; Reiner, J.; Chang, J.; Zhao, K. Oxidative cyclization of aldazines with bis(trifluoroacetoxy)iodobenzene. Tetrahedron Lett. 2005, 46, 2701–2704. [Google Scholar] [CrossRef]
- Cristian, D.; Paraschivescu, C.C.; Dumitru, I.; Matache, M.; Baciu, I.; Rută, L.L. Convenient preparation of unsymmetrical 2,5-disubstituted 1,3,4-oxadiazoles promoted by Dess–Martin reagent. Tetrahedron Lett. 2009, 50, 1886–1888. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).