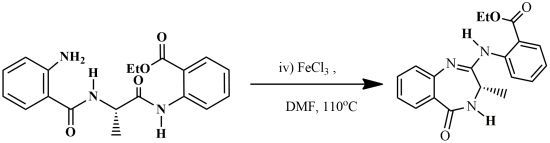
Ethyl 2-(3-Methyl-5-oxo-4,5-dihydro-3H-benzo[e][1,4]diazepin-2-ylamino)benzoate
Abstract
:Introduction
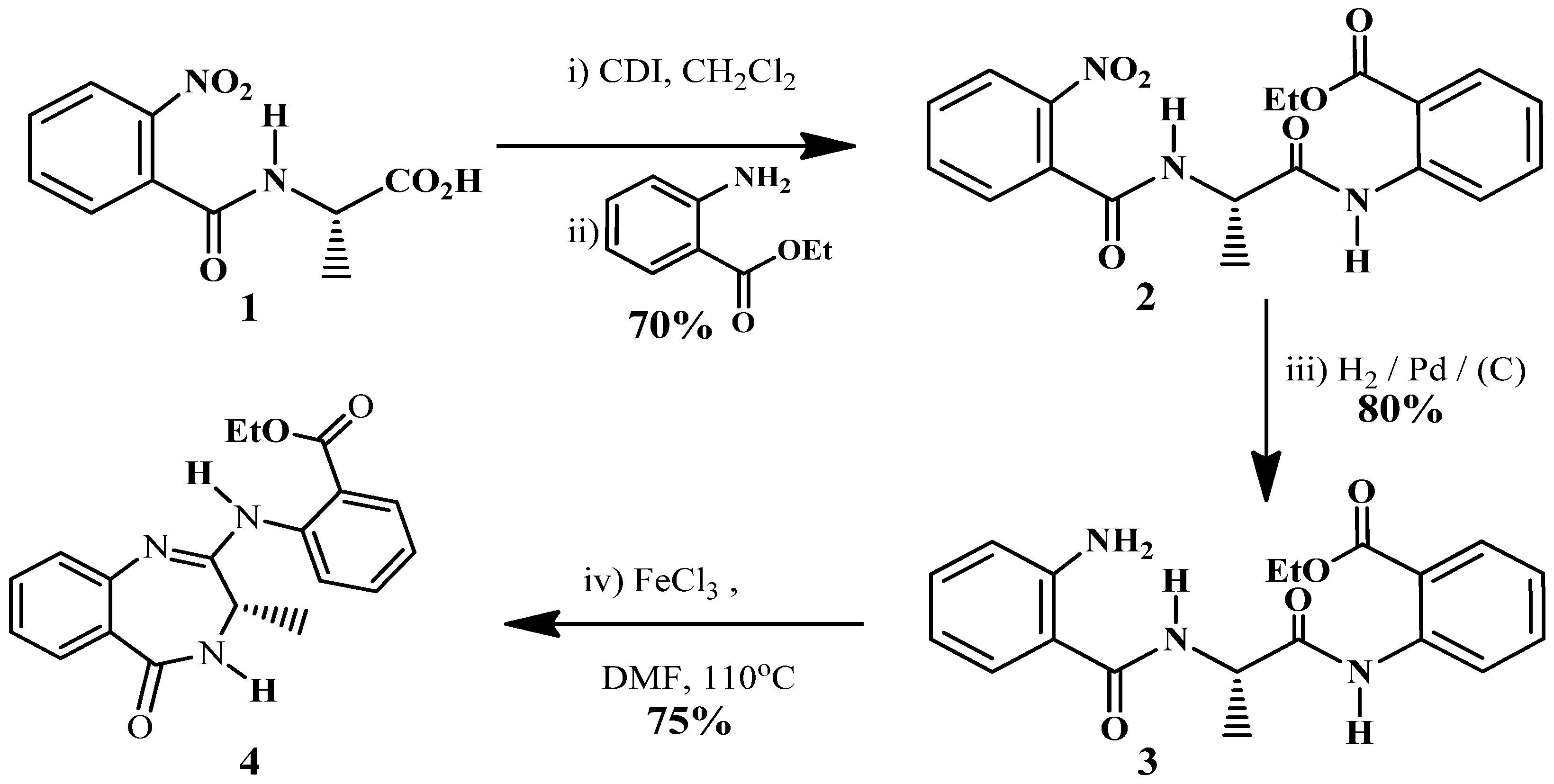
Results and Discussion
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Conflicts of Interest
References
- Ye, N.; Neumeyer, J.L.; Baldessarini, R.J.; Xuechu, Z.; Ao, Z. Update 1 of: Recent progress in development of dopamine receptor subtype-selective agents: Potential therapeutics for neurological and psychiatric disorders. Chem. Rev. 2013, 113, 123–178. [Google Scholar] [CrossRef] [PubMed]
- Verdié, P.; Subra, G.; Feliu, L.; Sanchez, P.; Bergé, G.; Garcin, G.; Martinez., J. on-Line synthesis of pseudopeptide library incorporating a benzodiazepinone turn mimic: Biological Evaluation on MC1 Receptors. J. Comb. Chem. 2007, 9, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Khoury, K.; Chanas, T.; Domling, A. Multicomponenet synthesis of diverse 1,4-benzodiazepine scaffolds. Org. Lett. 2012, 14, 5916–5919. [Google Scholar] [CrossRef] [PubMed]
- Hadjipavlou, L.D.; Hansch, C. Quantitative structure-activity relationships of benzodiazepines. Chem. Rev. 1994, 94, 1483–1505. [Google Scholar] [CrossRef]
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Sardina, F.J.; Rapoport, H. Enantionspecific synthesis of heterocycles from α-amino acids. Chem. Rev. 1996, 96, 1825–1872. [Google Scholar] [CrossRef] [PubMed]
- Al-Said, N.H. Effective formal synthesis of benzomalvin A. Monatsh. Chem. 2010, 141, 1249–1251. [Google Scholar] [CrossRef]
- Al-Said, N.H.; Shawakfeh, K.Q.; Ibrahim, M.I.; Tayyem, S.H. A facile synthesis of quinazolino[1,4]benzodiazepine alkaloids via reductive N-heterocyclization of N-(2-nitrobenzoyl)amides: Total synthesis of asperlicin C, circumdatin H, and analogues. ARKIVOC 2010, ix, 282–292. [Google Scholar]
- Taher, D.; Ishtaiwi, Z.N.; Al-Said, N.H. Efficient protocol to quinazolino[3,2-d][1,4]benzodiazepine-6,9-dione via Staudinger-aza-Wittig cyclizationapplication to synthesis of Asperlicin D. ARKIVOC 2008, xvi, 154–164. [Google Scholar]
- Hoffmann, E.; Jagnicinski, B. The formation of lactams of N-(2-amino)benzoylamino acids. J. Heterocyclic. Chem. 1966, 3, 348–351. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Said, N.H.; Al-Sghair, A.M. Ethyl 2-(3-Methyl-5-oxo-4,5-dihydro-3H-benzo[e][1,4]diazepin-2-ylamino)benzoate. Molbank 2013, 2013, M811. https://doi.org/10.3390/M811
Al-Said NH, Al-Sghair AM. Ethyl 2-(3-Methyl-5-oxo-4,5-dihydro-3H-benzo[e][1,4]diazepin-2-ylamino)benzoate. Molbank. 2013; 2013(4):M811. https://doi.org/10.3390/M811
Chicago/Turabian StyleAl-Said, Naim H., and Ayat M. Al-Sghair. 2013. "Ethyl 2-(3-Methyl-5-oxo-4,5-dihydro-3H-benzo[e][1,4]diazepin-2-ylamino)benzoate" Molbank 2013, no. 4: M811. https://doi.org/10.3390/M811
APA StyleAl-Said, N. H., & Al-Sghair, A. M. (2013). Ethyl 2-(3-Methyl-5-oxo-4,5-dihydro-3H-benzo[e][1,4]diazepin-2-ylamino)benzoate. Molbank, 2013(4), M811. https://doi.org/10.3390/M811