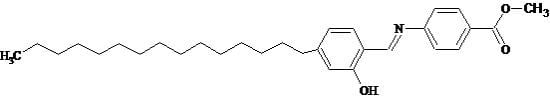
4-[(2-Hydroxy-4-pentadecyl-benzylidene)-amino]-benzoic Acid Methyl Ester
Abstract
:Introduction
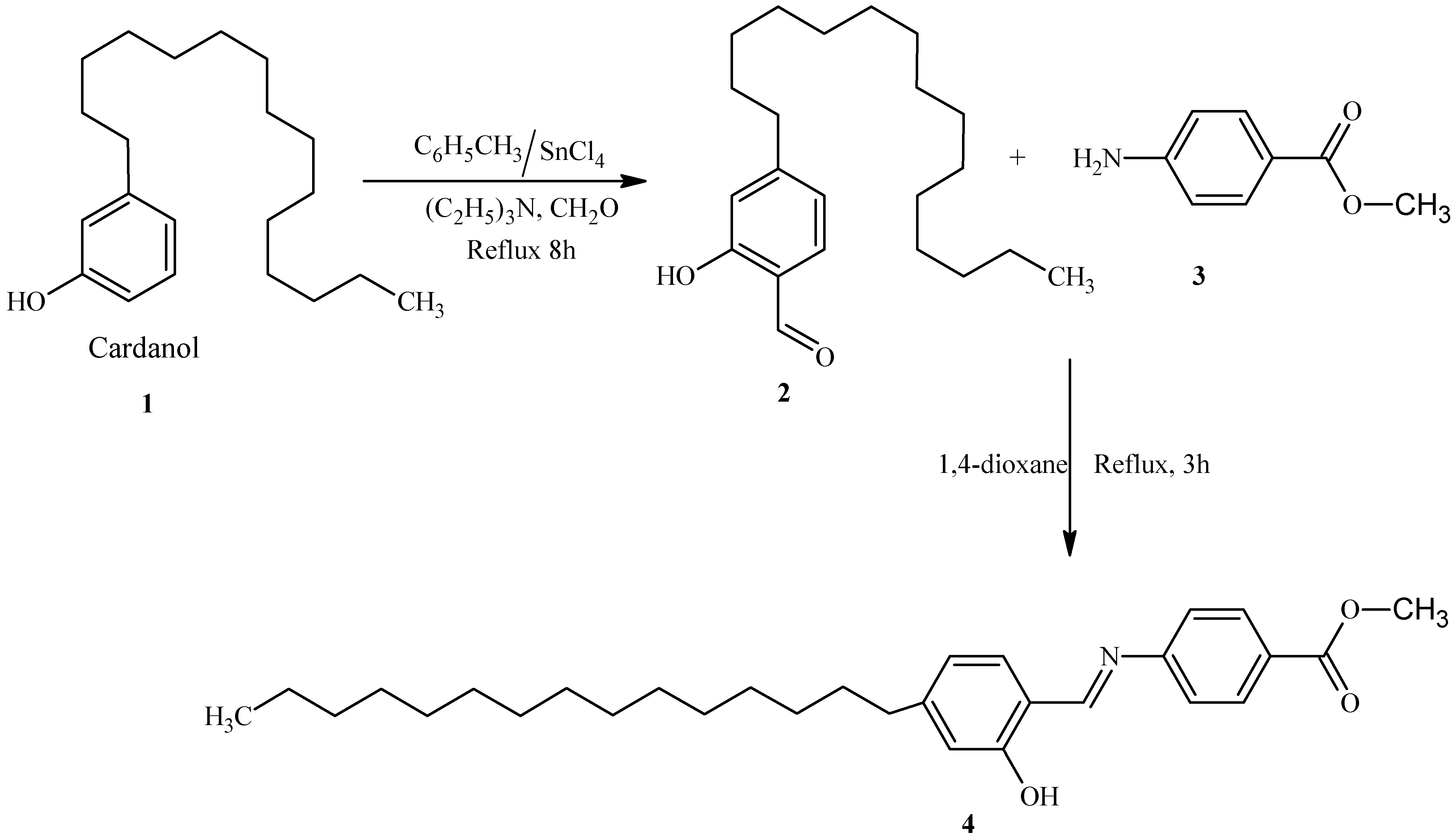
Synthesis
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Conflicts of Interest
References
- Hadjoudis, E.; Vittorakis, M. Moustakali-Mavridis, I. Photochromism and thermochromism of schiff bases in the solid state and in rigid glasses. Tetrahedron 1987, 43, 1345–1360. [Google Scholar] [CrossRef]
- Hadjoudis, E.; Rontoyianni, A.; Ambroziak, K.; Dziembowska, T.; Mavridis, I.M. Photochromism and thermochromism of solid trans-N,N′-bis(salicylidene)-1,2-cyclohexanediamines and trans-N,N′-bis-(2-hydroxynaphylidene)-1,2-cyclohexanediamine. J. Photochem. Photobiol. A Chem. 2004, 162, 521–530. [Google Scholar] [CrossRef]
- Oshima, A.; Momotake, A.; Arai, T. Photochromism, thermochromism, and solvatochromism of naphthalene-based analogues of salicylideneaniline in solution. J. Photochem. Photobiol. A Chem. 2004, 162, 473–479. [Google Scholar] [CrossRef]
- Yeap, G.Y.; Ha, S.T.; Ishizawa, N.; Suda, K.; Boey, P.L.; Mahmood, W.A.K. Synthesis, crystal structure and spectroscopic study of para substituted 2-hydroxy-3-methoxybenzalideneanilines. J. Mol. Struct. 2003, 658, 87–99. [Google Scholar] [CrossRef]
- Ha, S.T.; Ong, L.K.; Wong, J.P.W.; Yeap, G.Y.; Lin, H.C.; Ong, S.T.; Koh, T.M. Mesogenic Schiff’s base ether with dimethylamino end group. Phase Transit. 2009, 82, 387–397. [Google Scholar] [CrossRef]
- Ha, S.T.; Ong, L.K.; Ong, S.T.; Yeap, G.Y.; Wong, J.P.W.; Koh, T.M.; Lin, H.C. Synthesis and mesomorphic properties of new Schiff base esters with different alkyl chains. Chin. Chem. Lett. 2009, 20, 767–770. [Google Scholar] [CrossRef]
- Payne, P.; Tyman, J.H.P.; Mehet, S.K.; Ninagawa, A. The synthesis of 2-hydroxymethyl derivatives of phenols. J. Chem. Res. 2006, 37, 402–405. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Naganagowda, G.; Mahato, S.K.; Meijboom, R.; Petsom, A. 4-[(2-Hydroxy-4-pentadecyl-benzylidene)-amino]-benzoic Acid Methyl Ester. Molbank 2013, 2013, M810. https://doi.org/10.3390/M810
Naganagowda G, Mahato SK, Meijboom R, Petsom A. 4-[(2-Hydroxy-4-pentadecyl-benzylidene)-amino]-benzoic Acid Methyl Ester. Molbank. 2013; 2013(4):M810. https://doi.org/10.3390/M810
Chicago/Turabian StyleNaganagowda, Gadada, Sanjit Kumar Mahato, Reinout Meijboom, and Amorn Petsom. 2013. "4-[(2-Hydroxy-4-pentadecyl-benzylidene)-amino]-benzoic Acid Methyl Ester" Molbank 2013, no. 4: M810. https://doi.org/10.3390/M810
APA StyleNaganagowda, G., Mahato, S. K., Meijboom, R., & Petsom, A. (2013). 4-[(2-Hydroxy-4-pentadecyl-benzylidene)-amino]-benzoic Acid Methyl Ester. Molbank, 2013(4), M810. https://doi.org/10.3390/M810