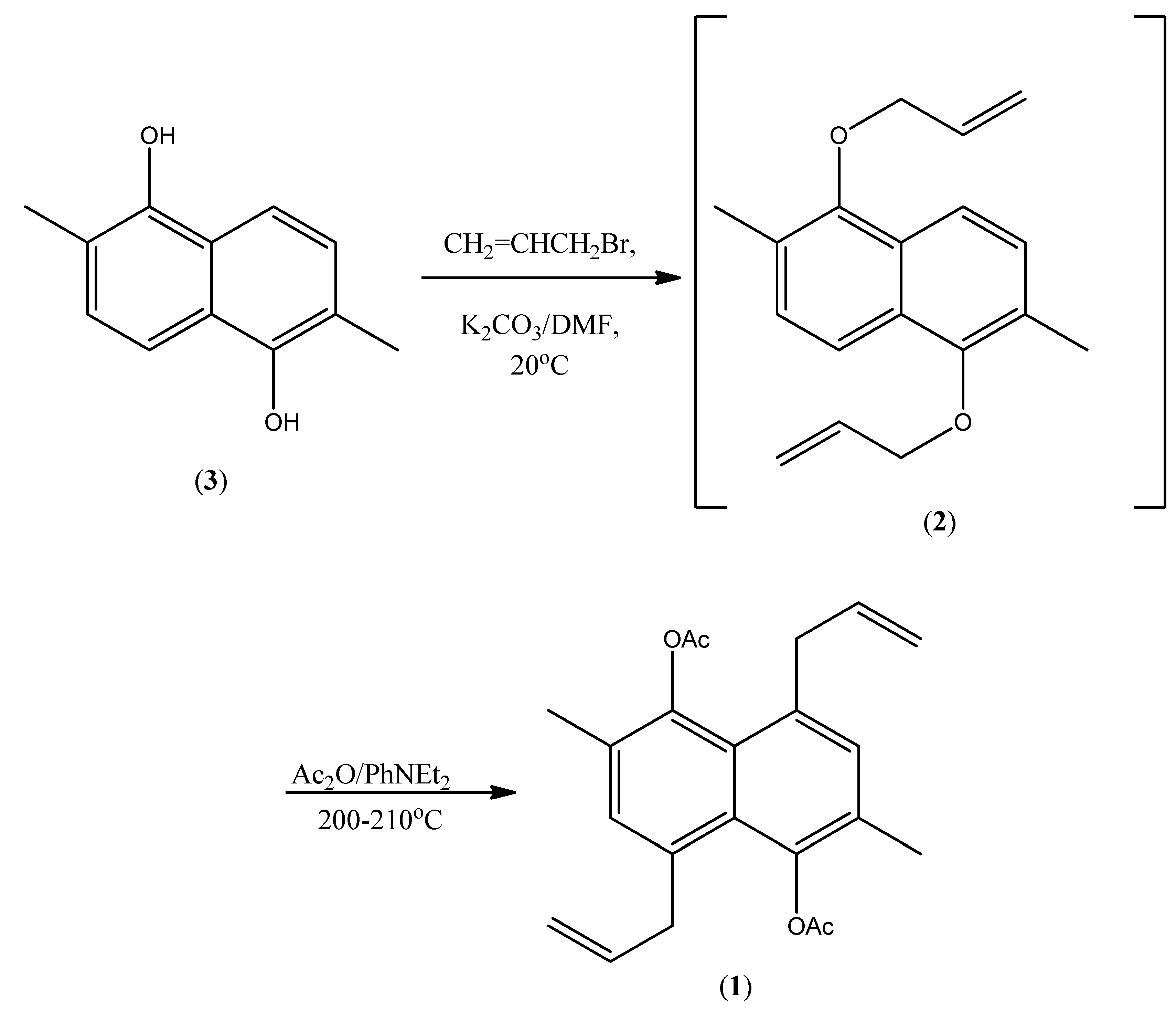
4,8-Bisallyl-2,6-dimethylnaphthalene-1,5-diyl Diacetate
Abstract
:Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
Conflict of Interest
References
- Balasubramaniyan, V. Peri interaction in naphthalene derivatives. Chem. Rev. 1966, 66, 567–641. [Google Scholar] [CrossRef]
- Imashiro, F.; Takegoshi, K.; Saika, A.; Taira, Z.; Asahi, Y. Molecular structure of 1,4,5,8-tetramethylnaphthalene and in-plane molecular rotation of some methyl-substituted naphthalenes in solids. J. Am. Chem. Soc. 1985, 107, 2341–2346. [Google Scholar] [CrossRef]
- Sylvester-Hvid, K.; Sørensen, J.; Schaumburg, K.; Bechgaard, K.; Christensen, J.B. Preparation of some 4,8-Dimethoxy-diformylnaphthalenes. Synth. Commun. 1993, 23, 1905–1914. [Google Scholar] [CrossRef]
- Christensen, J.B.; Johannsen, I.; Bechgaard, K. Synthesis and properties of substituted 1,6-dioxapyrene donors. J. Org. Chem. 1991, 56, 7055–7058. [Google Scholar] [CrossRef]
- Mortensen, M.B.; Schlüter, A.; Sørensen, J.; Christensen, J.B. A convenient preparation of 1,6-dioxapyrene-2,7-dicarboxylic acid diethylester. Acta Chem. Scand. 1997, 51, 807–809. [Google Scholar] [CrossRef]
- Christensen, J.B.; Bechgaard, K. The chemistry of 1,6-dioxapyrenes part 3 [1,2]: Scope and limitations of an acid catalyzed ring-closing reaction. J. Het. Chem. 2003, 40, 757–761. [Google Scholar] [CrossRef]
- Tyson, D.S.; Fabrizio, E.F.; Panzner, M.J.; Kinder, J.D.; Buisson, J.-P.; Christensen, J.B.; Meador, M.A. Synthesis, characterization, and optical properties of a cyano-functionalized 4,5,9,10-tetraaryl-1,6-dioxapyrene. J. Photochem. Photobiol. A 2005, 172, 97–107. [Google Scholar] [CrossRef]
- Tilak, B.D. Synthesis of thiophenes and thiapyrans. IV. Thiophenes and thiapyrans from naphthalenethiols. Proc. Indian Acad. Sci. 1951, 33A, 71–77. [Google Scholar]
- Nakasuji, K.; Kubota, H.; Kotani, T.; Murata, I.; Saito, G.; Enoki, T.; Imaeda, K.; Inokuchi, H.; Honda, M.; Katayama, C.; et al. Novel peri-condensed Weitz-type donors: Synthesis, physical properties, and crystal structures of 3,10-dithiaperylene (DTPR), 1,6-dithiapyrene (DTPY), and some of their CT complexes. J. Am. Chem. Soc. 1986, 108, 3460–3466. [Google Scholar] [CrossRef]
- Nakasuji, K.; Sasaki, M.; Kotani, T.; Murata, I.; Enoki, T.; Imaeda, K.; Inokuchi, H.; Kawamoto, A.; Tanaka, J. Methylthio- and ethanediyldithio-substituted 1,6-dithiapyrenes and their charge-transfer complexes: New organic molecular metals. J. Am. Chem. Soc. 1987, 109, 6970–6975. [Google Scholar] [CrossRef]
- Miyazaki, E.; Morita, Y.; Nakasuji, K. 2-Iodo-1,6-dithiapyrene: Syntheses, crystal structures and physical properties of CT complexes and salt. Polyhedron 2005, 24, 2632–2638. [Google Scholar] [CrossRef]
- Tarbell, D.S. The Claisen Rearrangement. In Organic Reactions; Wiley: New York, NY, USA, 1944; Volume 2, pp. 1–48. [Google Scholar]
- Fieser, L.F.; Campbell, W.P.; Fry, E.M. Synthesis of quinones related to Vitamins K1 and K2. J. Am. Chem. Soc. 1939, 61, 2206–2218. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Christensen, J.B.; Sørensen, J.N.; Schaumburg, K.; Bechgaard, K. 4,8-Bisallyl-2,6-dimethylnaphthalene-1,5-diyl Diacetate. Molbank 2014, 2014, M812. https://doi.org/10.3390/M812
Christensen JB, Sørensen JN, Schaumburg K, Bechgaard K. 4,8-Bisallyl-2,6-dimethylnaphthalene-1,5-diyl Diacetate. Molbank. 2014; 2014(1):M812. https://doi.org/10.3390/M812
Chicago/Turabian StyleChristensen, Jørn B., Jeanett N. Sørensen, Kjeld Schaumburg, and Klaus Bechgaard. 2014. "4,8-Bisallyl-2,6-dimethylnaphthalene-1,5-diyl Diacetate" Molbank 2014, no. 1: M812. https://doi.org/10.3390/M812
APA StyleChristensen, J. B., Sørensen, J. N., Schaumburg, K., & Bechgaard, K. (2014). 4,8-Bisallyl-2,6-dimethylnaphthalene-1,5-diyl Diacetate. Molbank, 2014(1), M812. https://doi.org/10.3390/M812





