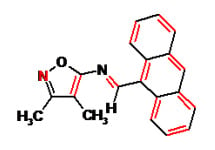
Anthracen-9-ylmethylene-(3,4-dimethylisoxazol-5-yl)amine
Abstract
: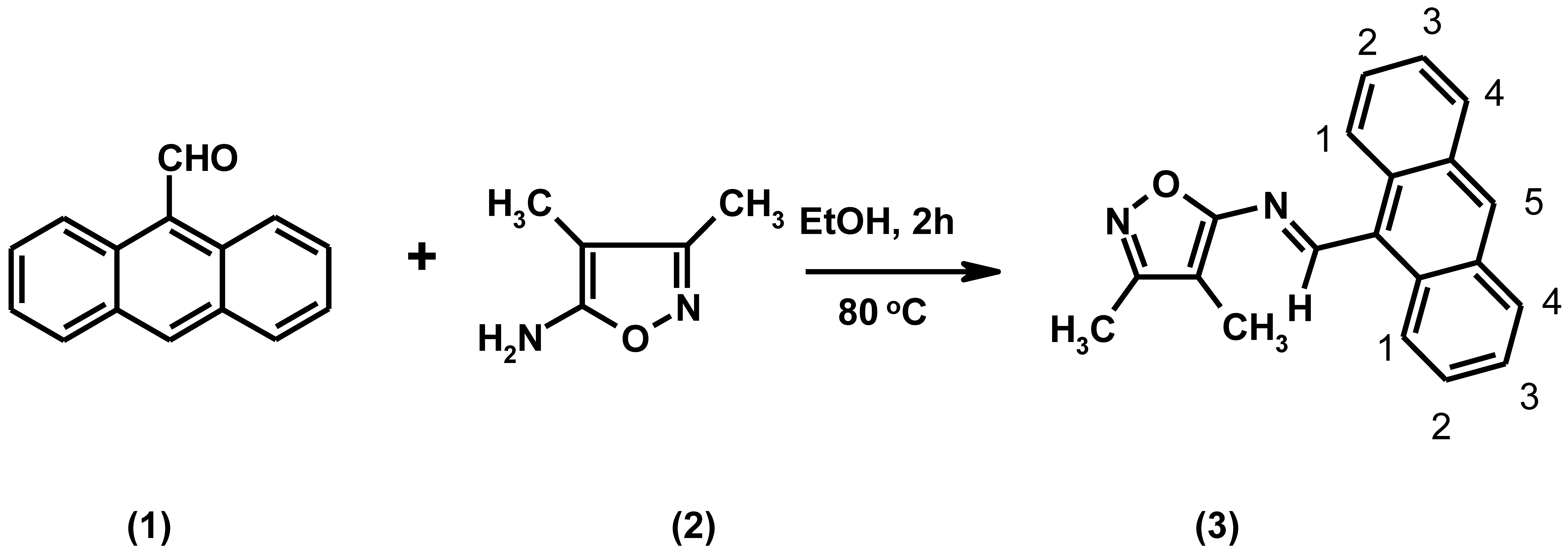
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Chauhan, P.M.S.; Martins, C.J.A.; Horwell, D.C. Syntheses of novel heterocycles as anticancer agents. Bioorg. Med. Chem. 2005, 13, 3513–3518. [Google Scholar] [CrossRef] [PubMed]
- Raffa, D.; Daidone, G.; Maggio, B.; Schillaci, D.; Plescia, F.; Torta, L. Synthesis and antifungal activity of new N-isoxazolyl-2-iodobenzamides. Il Farmaco 1999, 54, 90–94. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Photophysics, photochemistry and photobiology of curcumin: Studies from organic solutions, bio-mimetics and living cells. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Shakru, P.; Subhashini, N.J.P.; Kumar, S.; Shivaraj, K. Synthesis, characterization and antimicrobial studies on Cobalt (II),Nickel (II), Copper (II) and Zinc (II) complexes of N, O, S donor Schiff bases. J. Chem. Pharm. Res. 2010, 2, 38–46. [Google Scholar]
- Bhuiyan, M.D.H.; Teshome, A.; Gainsford, G.J.; Ashraf, M.; Clays, K.; Asselberghs, I.; Kay, A.J. Synthesis, characterization, linear and non-linear optical (NLO) properties of some Schiff’s bases. Opt. Mater. 2010, 32, 669–672. [Google Scholar] [CrossRef]
- Rudresha, B.J.; Bhat, B.R.; Kumar, H.C.S.; Kumar, K.I.S.; Safakath, K.; Philip, R. Synthesis, characterization and third-order nonlinear optical studies of copper complexes containing 1,10-phenanthroline-5,6-dione and triphenylphosphine ligands. Synthetic Met. 2011, 161, 535–539. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhatnagar, J.M. Copper(II) selective electrochemical sensor based on Schiff Base complexes. Talanta 2004, 64, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Asiri, A.M.; Khan, S.A.; Rasl, M.G. N-[(9-Ethyl-9H-carbazol-3-yl)methylene]-3,4-dimethylisoxazol-5-amine. Molbank 2010, 2011, M684. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Asiri, A.M.; Khan, S.A. Anthracen-9-ylmethylene-(3,4-dimethylisoxazol-5-yl)amine. Molbank 2011, 2011, M736. https://doi.org/10.3390/M736
Asiri AM, Khan SA. Anthracen-9-ylmethylene-(3,4-dimethylisoxazol-5-yl)amine. Molbank. 2011; 2011(3):M736. https://doi.org/10.3390/M736
Chicago/Turabian StyleAsiri, Abdullah M., and Salman A. Khan. 2011. "Anthracen-9-ylmethylene-(3,4-dimethylisoxazol-5-yl)amine" Molbank 2011, no. 3: M736. https://doi.org/10.3390/M736
APA StyleAsiri, A. M., & Khan, S. A. (2011). Anthracen-9-ylmethylene-(3,4-dimethylisoxazol-5-yl)amine. Molbank, 2011(3), M736. https://doi.org/10.3390/M736