2-tert-Butyl-5,6,7,8,9,10-hexahydrocyclohepta[b]indole
Abstract
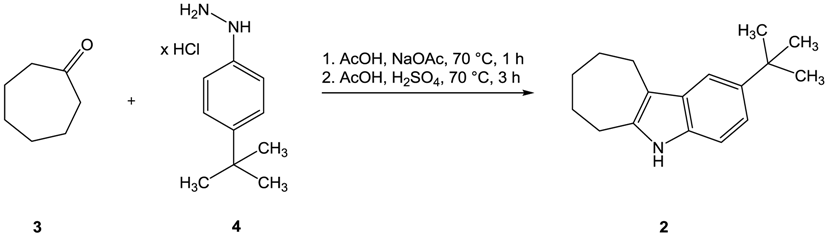
:
Experimental
General
2-tert-Butyl-5,6,7,8,9,10-hexahydrocyclohepta[b]indole
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References and Notes
- Kunick, C.; Zeng, Z.; Gussio, R.; Zaharevitz, D.; Leost, M.; Totzke, F.; Schächtele, C.; Kubbutat, M.H.G.; Meijer, L.; Lemcke, T. Structure-aided optimization of kinase inhibitors derived from alsterpaullone. ChemBioChem 2005, 6, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Lahusen, T.; De Siervi, A.; Kunick, C.; Senderowicz, A.M. Alsterpaullone, a novel cyclin-dependent kinase inhibitor, induces apoptosis by activation of caspase 9 due to perturbation in mitochondrial membrane potential. Mol. Carcinog. 2003, 36, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Pies, T.; Schaper, K.-J.; Leost, M.; Zaharevitz, D.W.; Gussio, R.; Meijer, L.; Kunick, C. CDK1-inhibitory activity of paullones depends on electronic properties of 9-substituents. Arch. Pharm. Pharm. Med. Chem. 2004, 337, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Wieking, K.; Knockaert, M.; Leost, M.; Zaharevitz, D.W.; Meijer, L.; Kunick, C. Synthesis of paullones with aminoalkyl side chains. Arch. Pharm. Pharm. Med. Chem. 2002, 335, 311–317. [Google Scholar] [CrossRef]
- Xie, X.; Lemcke, T.; Gussio, R.; Zaharevitz, D.W.; Leost, M.; Meijer, L.; Kunick, C. Epoxide-containing side chains enhance antiproliferative activity of paullones. Eur. J. Med. Chem. 2005, 40, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Stukenbrock, H.; Mussmann, R.; Geese, M.; Ferandin, Y.; Lozach, O.; Lemcke, T.; Kegel, S.; Lomow, A.; Burk, U.; Dohrmann, C.; Meijer, L.; Austen, M.; Kunick, C. 9-Cyano-1-azapaullone (cazpaullone), a glycogen synthase kinase-3 (GSK-3) inhibitor activating pancreatic beta cell protection and replication. J. Med. Chem. 2008, 51, 2196–2207. [Google Scholar] [CrossRef] [PubMed]
- Reichwald, C.; Shimony, O.; Dunkel, U.; Sacerdoti-Sierra, N.; Jaffe, C.L.; Kunick, C. 2-(3-Aryl-3-oxopropen-1-yl)-9-tert-butyl-paullones: A new antileishmanial chemotype. J. Med. Chem. 2008, 51, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, G.R.; Kuethe, J.T. Practical methodologies for the synthesis of indoles. Chem. Rev. 2006, 106, 2875–2911. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.L. Progress in the Fischer indole reaction. A review. Org. Prep. Proced. Int. 1993, 25, 607–632. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Falke, H.; Bumiller, K.; Harbig, S.; Masch, A.; Wobbe, J.; Kunick, C. 2-tert-Butyl-5,6,7,8,9,10-hexahydrocyclohepta[b]indole. Molbank 2011, 2011, M737. https://doi.org/10.3390/M737
Falke H, Bumiller K, Harbig S, Masch A, Wobbe J, Kunick C. 2-tert-Butyl-5,6,7,8,9,10-hexahydrocyclohepta[b]indole. Molbank. 2011; 2011(3):M737. https://doi.org/10.3390/M737
Chicago/Turabian StyleFalke, Hannes, Katharina Bumiller, Sabrina Harbig, Andreas Masch, Janina Wobbe, and Conrad Kunick. 2011. "2-tert-Butyl-5,6,7,8,9,10-hexahydrocyclohepta[b]indole" Molbank 2011, no. 3: M737. https://doi.org/10.3390/M737
APA StyleFalke, H., Bumiller, K., Harbig, S., Masch, A., Wobbe, J., & Kunick, C. (2011). 2-tert-Butyl-5,6,7,8,9,10-hexahydrocyclohepta[b]indole. Molbank, 2011(3), M737. https://doi.org/10.3390/M737




