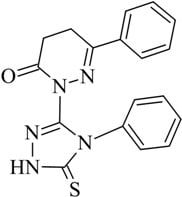
6-Phenyl-2-(4-phenyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)-4,5-dihydropyridazin-3(2H)-one
Abstract
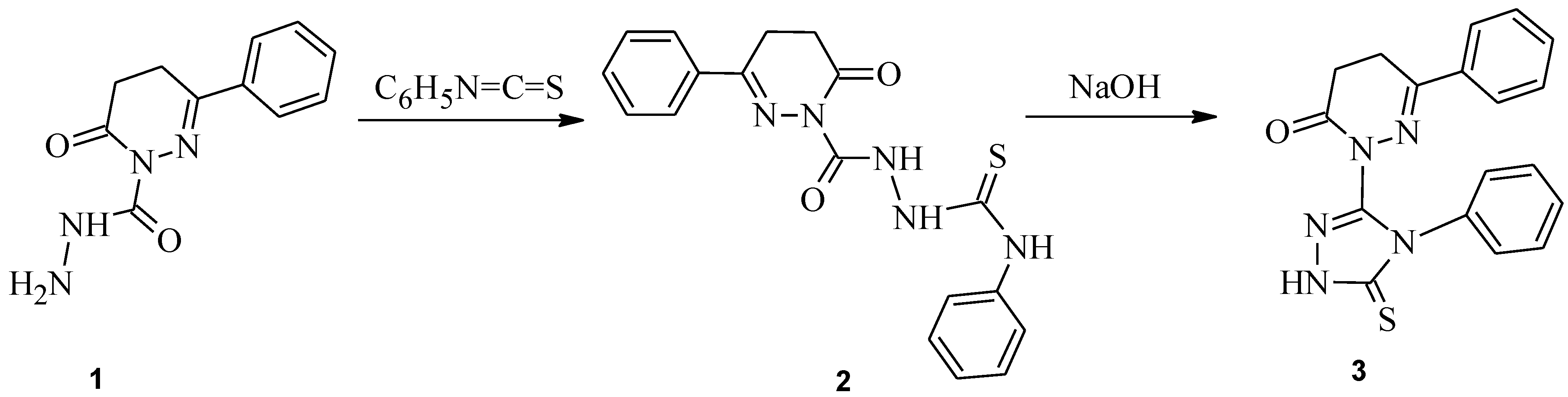
:Synthesis of 6-phenyl-2-(4-phenyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)-4,5-dihydropyridazin-3(2H)-one 3
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Coelho, A.; Sotelo, E.; Ravina, E. Pyridazine derivatives. Part 33: Sonogashira approaches in the synthesis of 5-substituted-6-phenyl-3(2H)-pyridazinones. Tetrahedron 2003, 59, 2477–2484. [Google Scholar] [CrossRef]
- Demirayak, S.; Karaburun, A.C.; Beis, R. Some pyrrole substituted aryl pyridazinone and phthalazinone derivatives and their antihypertensive activities. Eur. J. Med. Chem. 2004, 39, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Mishra, R.; Shaharyar, M. Synthesis, characterization and antihypertensive activity of pyridazinone derivatives. Eur. J. Med. Chem. 2010, 45, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Monge, A.; Parrado, P.; Font, M.; Alvarez, E.F. Selective thromboxane synthetase inhibitors and antihypertensive agents. New derivatives of 4-hydrazino-5H-pyridazino[4,5-b]indole, 4-hydrazinotriazino[4,5-a]indole, and related compounds. J. Med. Chem. 1987, 30, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Rubat, C.; Coudert, P.; Refouvelet, B.; Tronche, P.; Bastide, P. Anticonvulsant activity of 3-oxo-5-substituted benzylidene-6-methyl-(4H)-2-pyridazinylacetamides and 2-pyridazinylacetyl-hydrazides. Chem. Pharm. Bull. 1990, 38, 3009–3013. [Google Scholar] [CrossRef] [PubMed]
- Sircar, I.; Weishaar, R.E.; Kobylarz, D.; Moos, W.H.; Bristol, J.A. Cardiotonic agents. Inhibition of separated forms of cyclic nucleotide phosphodiesterase from guinea pig cardiac muscle by 4,5-dihydro-6-[4-(1H-imidazol-1-yl)phenyl]-3(2H)-pyridazinones and related compounds. Structure-activity relationships and correlation with in vivo positive inotropic activity. J. Med. Chem. 1987, 30, 1955–1962. [Google Scholar] [PubMed]
- Longo, J.G.; Verde, I.; Castro, M.E. Pyridazine derivatives XIV. Study of the vasorelaxant action of 6-aryl-5-piperidino-3-hydrazinopyridazines in isolated rat thoracic aorta: Comparison with hydralazine. J. Pharm. Sci. 1993, 82, 286–290. [Google Scholar]
- Akahane, A.; Katayama, H.; Mitsunaga, T. Discovery of 6-oxo-3-(2-phenylpyrazolo[1,5-a]pyridin-3-yl)-1(6H)-pyridazinebutanoic acid (FK 838): A novel non-xanthine adenosine A1 receptor antagonist with potent diuretic activity. J. Med. Chem. 1999, 42, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Livermone, D.G.H.; Bethell, R.C.; Cammack, N. Synthesis and anti-HIV-1 activity of a series of imidazo[1,5-b]pyridazines. J. Med. Chem. 1993, 36, 3784–3794. [Google Scholar] [CrossRef]
- Malinka, W.; Redzicka, A.; Lozach, O. New derivatives of pyrrolo[3,4-d]pyridazinone and their anticancer effects. Il Farmaco 2004, 59, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Abouzid, K.; Hakeem, M.A.; Khalil, O.; Maklad, Y. Pyridazinone derivatives: Design, synthesis, and in vitro vasorelaxant activity. Bioorg. Med. Chem. 2008, 16, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Combs, D.W.; Rampulla, M.S.; Bell, S.C.; Klaubert, D.H.; Tobia, A.J.; Falotico, R.; Haertlein, B.; Weiss, C.L.; Moore, J.B. 6-Benzoxazinylpyridazin-3-ones: Potent, long-acting positive inotrope and peripheral vasodilator agents. J. Med. Chem. 1990, 22, 380–386. [Google Scholar] [CrossRef]
- Robertson, D.W.; Jones, N.D.; Krushinski, J.H.; Pollock, G.D.; Swartzendruber, J.K.; Hayes, J.S. Molecular structure of the dihydropyridazinone cardiotonic 1,3-dihydro-3,3-dimethyl-5-(1,4,5,6-tetrahydro-6-oxo-3-pyridazinyl)-2H-indol-2-one, a potent inhibitor of cyclic AMP phospho-diesterase. J. Med. Chem. 1987, 30, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Archan, S.; Toller, W. Levosimendan: current status and future prospects. Curr. Opin. Anesthesiol. 2008, 21, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Siddiqui, A.A.; Kumar, R.; Kumar, S. 6-Oxo-3-phenyl-5,6-dihydropyridazine-1(4H)-carbohydrazide. Molbank 2010, 2010, M652. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mishra, R.; Siddiqui, A.A.; Shaharyar, M.; Husain, A.; Rashid, M. 6-Phenyl-2-(4-phenyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)-4,5-dihydropyridazin-3(2H)-one. Molbank 2010, 2010, M700. https://doi.org/10.3390/M700
Mishra R, Siddiqui AA, Shaharyar M, Husain A, Rashid M. 6-Phenyl-2-(4-phenyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)-4,5-dihydropyridazin-3(2H)-one. Molbank. 2010; 2010(4):M700. https://doi.org/10.3390/M700
Chicago/Turabian StyleMishra, Ravinesh, Anees A. Siddiqui, Mohammad Shaharyar, Asif Husain, and Mohd Rashid. 2010. "6-Phenyl-2-(4-phenyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)-4,5-dihydropyridazin-3(2H)-one" Molbank 2010, no. 4: M700. https://doi.org/10.3390/M700
APA StyleMishra, R., Siddiqui, A. A., Shaharyar, M., Husain, A., & Rashid, M. (2010). 6-Phenyl-2-(4-phenyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)-4,5-dihydropyridazin-3(2H)-one. Molbank, 2010(4), M700. https://doi.org/10.3390/M700