Abstract
A practical synthesis of N-cyclohexyl-11-(octylthio)undecanamide by thiol-ene click coupling reaction under UV light irradiation is reported. The title compound was characterized by elemental analysis, FT-IR, 1H NMR, 13C NMR and MS spectroscopic methods. This molecule was found to be an efficient gelator for fluid oils, and the main physical parameters of the formed gels were also examined.
In 2001, Sharpless and co-workers introduced the invasive concept of “click” chemistry to describe a modular approach towards a synthesis that uses only the most practical chemical transformations to make molecular connections with excellent fidelity [1]. Among others, the century-old addition of thiols to alkenes [2] (thiol-ene coupling, TEC) is emerging as an attractive click process mainly due to its highly efficient and orthogonal nature to a wide range of functional groups, robustness of the formed anti-Markovnikov-type thioether linkage, and the compatibility of the ligation process with water and oxygen [3]. As has occurred with other click-type reactions [4], the above-mentioned free-radical photochemically/thermally-induced version of TEC has also found great applicability in the synthesis of functional soft materials such as organo- and hydrogels [5,6].
Herein, we report the practical preparation of the low-molecular-weight gelator N-cyclohexyl-11-(octylthio)undecanamide (4) by thiol-ene click coupling reaction under UV light irradiation (Figure 1 left). Alkene precursor 1 was easily prepared by DCC-coupling between cylohexylamine and the corresponding acid derivative in CH2Cl2. The title compound 4 was characterized by elemental analysis, FT-IR, 1H NMR, 13C NMR and MS spectroscopic methods (see Experimental Section). Interestingly, 4 was found to act as an efficient gelator for fluid oils (Figure 1 right, vials A−C) through molecular self-assembly driven by non-covalent interactions. The minimum gelation concentrations (MGC) were found to be lower than 1 wt.%, and the gel-to-sol transition temperatures (Tgel) in the range of 27−39 °C. As expected, Tgel values were found to increase with the concentration of the gelator probably due to the formation of more closely packed 3D-networks [7]. All gels were stable for several weeks below their Tgel, and showed a thermoreversible response that was reproducible over several heating/cooling cycles. A novel ionogel made of 4 in ionic liquid C10MIMCl was also successfully prepared (Figure 1 right, vial D).
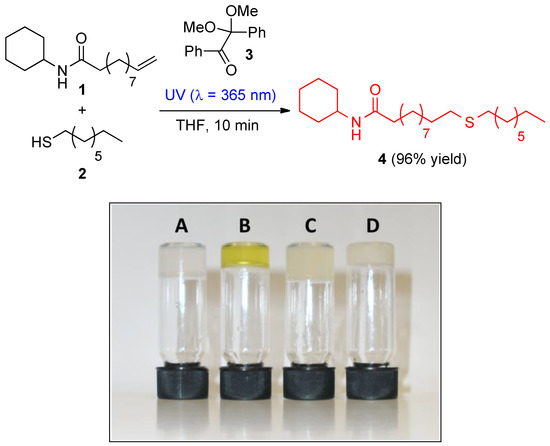
Figure 1.
Synthesis of gelator N-cyclohexyl-11-(octylthio)undecanamide (4) and gels made in silicone oil (vial A, MGC = 0.5 wt.%, Tgel = 39 ± 3 °C), olive oil (vial B, MGC = 0.8 wt.%, Tgel = 27 ± 3 °C) [8], rapeseed oil (vial C, MGC = 0.8 wt.%, Tgel = 27 ± 3 °C) and ionic liquid C10MIMCl (vial D, MGC = 0.7 wt.%, Tgel = 44 ± 3 °C).
Table 1 outlines the outcome of the TEC reaction between 1 and 2 under different experimental conditions chosen for comparative studies. The use of a UV-lamp (λ = 365 nm) as irradiation source, THF as solvent, and catalytic amounts of DMPA (3) as radical initiator, provided the best results (entry 6). In general, the use of an irradiation source of higher wavelength (i.e., near-UV LEDs, λ = 400 nm) also afforded the desired product 4 albeit in lower yield (ca. 47−65%).

Table 1.
Thiol-ene coupling reaction between 1 and 2 under different conditions.
Experimental
General
1H and 13C NMR spectra were recorded at 25 °C on a Bruker Avance 300 spectrometer in CDCl3 as solvent, and chemical shifts are reported relative to Me4Si (δ = 0). Low-resolution mass spectra were obtained by using a Varian MAT 311A spectrometer. Elemental analyses were performed on a Heraeus Mikro-Rapid analyzer. Infrared spectra were recorded on a Bio-Rad Excalibur FTS 3000 MX spectrophotometer. Melting points (mp) were measured in an Opti Melt MPA 100 and are uncorrected. Thin-layer chromatography was carried out on Merck Aluminium sheets coated with silica gel 60 F254; visualization by use of phosphomolybdic acid in ethanol with heating. Chromatographic purifications were conducted by column chromatography using silica gel (0.063−0.200 mm) obtained from Merck Compounds. A UV-Hand lamp (Spectroline ENB-280C, 8 W, 365/312 nm) was used for the experiments. All solvents were purified by standard techniques [8]. Rheological properties were determined by using a Bohlin CVO Rheometer. Microwave irradiation was performed in a single mode focused CEM Explorer Hybrid 12 reactor. Alkene precursor 1 is commercially available in milligram quantities from Aurora Fine Chemicals LLC and Princeton BioMolecular Research, Inc. The complete analytics for this compound can be found in the Supporting Files.
Synthesis of N-cyclohexyl-11-(octylthio)undecanamide (4): To a solution of N-cyclohexyl-10-undecenamide (1) (50 mg, 0.188 mmol) in THF (2 mL), octane-1-thiol (2) (0.098 mL, 0.564 mmol) and DMPA (3) (2.5 mg, 0.01 mmol) were added. The reaction mixture was stirred under 365 nm-UV irradiation for 10 min. At that time, TLC analysis showed full conversion of the starting material. The solvent was evaporated and the crude product was purified by column chromatography (n-hexane/ethyl acetate = 80/20) affording 4 (74.4 mg, 96% yield) as a white solid. From a practical stand point it is worth to mention that product 4 was found to precipitate in the reaction mixture when acetone, MeOH or DMSO were used as solvents. Characterization data for 4: TLC Rf (n-hexane/ethyl acetate = 70/30) = 0.4; mp = 83 ± 1 °C; 1H NMR (300 MHz, CDCl3) δ/ppm = 0.88 (t, J = 6.8 Hz, 3H), 1.08–1.14 (m, 2H), 1.26–1.40 (m, 24H), 1.52–1.62 (m, 8H), 1.69 (dt, J = 2.7 Hz, J = 3.0 Hz, 2H), 1.91 (dd, J = 3.6 Hz, J = 12.4 Hz, 2H), 2.12 (t, J = 6.0 Hz, 2H), 2.49 (t, J = 5.5 Hz, 4H), 3.77 (tdt, J = 3.9 Hz, J = 7.9 Hz, J = 11.9 Hz, 1H), 5.23 (brd, J = 8.3 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ/ppm = 14.1, 22.7, 24.9, 25.6, 25.9, 29.0, 29.2, 29.3, 29.4, 29.5, 29.7, 31.8, 32.2, 33.3, 37.1, 48.0, 172.1; FT-IR νmax (cm-1) 3296 (N−H stretching), 1636 (C=O stretching, amide I band), 1546 (N−H bending, amide II band), 1465, 1231, 623 (C−S−C stretching); MS (ESI) m/z 412 [MH+]. Elemental analysis calculated for C25H49NOS: C, 72.93; H, 12.00; N, 3.40; S. 7.79; found: C, 72.69; H, 12.02; N, 3.06; S. 7.51.
Gelation experiments and gel characterization
In a typical gelation experiment, a weighted amount of 4 and the appropriate oil (1 g) were placed in a screw-capped glass vial (4.5 cm length and 1.5 cm diameter) and heated with a heat gun until the solid was dissolved. The resulting isotropic solution was cooled down to room temperature affording gels within 15 minutes in silicone oil and ionic liquid (the other reported gels were formed by cooling down the solutions at 3 °C in the refrigerator). The soft materials were classified as gels if no gravitational flow was observed. Unless otherwise indicated, Tgel values were determined by the “dropping ball method” [9] (temperature rate = 1 °C min−1; steel ball: weight = 110 mg, Ø = 2 mm) and reported as the average of three random measurements. Tgel was defined herein as the temperature at which the ball reached the bottom of the glass vial. For the gel made of 4 in olive oil, Tgel was estimated as the temperature at which the gel moved on tilting of the vial when it was immersed in a heating silicone oil bath. Standard oscillatory rheology experiments further confirmed the gel nature of the samples (storage modulus (G') > loss modulus (G'')) [7].
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
We gratefully acknowledge the financial support rendered by Universität Regensburg and the Alexander von Humboldt Foundation. We thank König’s and Reiser’s research groups at Universität Regensburg for general assistance.
References and Notes
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Posner, T. Beiträge zur Kenntniss der ungesättigten Verbindungen. II. Ueber die Addition von Mercaptanen an ungesättigte Kohlenwasserstoffe. Ber. Dtsch. Chem. Ges. 1905, 38, 646–657. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol-ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573, and references therein. [Google Scholar] [CrossRef] [PubMed]
- Binder, W.H.; Sachsenhofer, R. ‘Click’ Chemistry in Polymer and Materials Science. Macromol. Rapid Commun. 2007, 28, 15–54. [Google Scholar] [CrossRef]
- Dondoni, A. The Emergence of Thiol-Ene Coupling as a Click Process for Materials and Bioorganic Chemistry. Angew. Chem. Int. Ed. 2008, 47, 8995–8997. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Lin, B.F.; Campos, L.M.; Dimitriou, M.D.; Hikita, S.T.; Treat, N.D.; Tirrell, M.V.; Clegg, D.O.; Kramer, E.J.; Hawker, C.J. A Versatile Approach to High-Throughput Microarrays Using Thiol-ene Chemistry. Nat. Chem. 2010, 2, 138–145, and references therein. [Google Scholar] [CrossRef] [PubMed]
- See Supplementary Files.
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1996. [Google Scholar]
- Esch, J.V.; De Feyter, S.; De Schryver, F.; Kellogg, R.M.; Feringa, B.L. Self-assembly of bisurea compounds in organic solvents and on solid substrates. Chem. Eur. J. 1997, 3, 1238–1243. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).