Abstract
A new sulfide, 2-methyl-7-(phenylsulfanylmethyl)naphthalene was synthesized and its MS, IR, 1H NMR, 13C NMR and DEPT-135 data are reported.
1. Introduction
In connection with our investigation of competitive mesolytic cleavages [1] of radical anions, we needed 2-methyl-7-(phenylsulfanylmethyl)naphthalene (3) as gas chromatography standard. The synthetic method of choice for this naphthylmethyl phenyl sulfide is reaction between naphthylmethyl halide and a corresponding thiophenoxide. From the various developed procedures [2,3,4,5,6,7] we have decided to utilize the phase-transfer variant used by Guthrie and Maslak [7] for preparation of the analogous ethers. The starting material for these derivatives was 2-bromomethyl-7-methylnaphthalene (1), which could be conveniently obtained by benzylic bromination of the commercially available 2,7-dimethylnaphthalene, using the procedure described by Buuhoi and co-workers [8]. We would like to report a convenient procedure for preparation of 2-methyl-7-(phenylsulfanylmethyl)naphthalene.
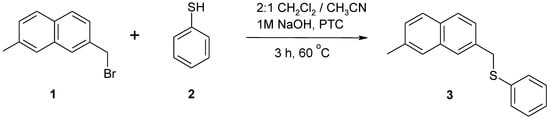
Scheme 1.
Synthesis of 2-methyl-7-(phenylsulfanylmethyl)naphthalene.
2. Experimental
2.1. General
All chemicals were obtained from commercial sources and were used without further purification. Melting points were determined using a Mel-Temp apparatus and are uncorrected. NMR spectra were recorded on a Bruker 400 MHz instrument, using deuteriochloroform as solvent and tetramethylsilane as internal standard. The IR spectra were recorded on a Perkin Elmer Model 1600 instrument between sodium chloride plates in carbon tetrachloride. The number of hydrogens on each carbon was determined from 13C NMR and DEPT-135 spectra. The mass spectra were recorded on a Kratos MS-25 RFA double-focusing mass spectrometer in electron impact (EI) mode. Gas chromatography was performed on a Varian 3700 instrument with packed column. The column was 1/8” in diameter and 50 cm in length packed with 5% OV-101 on supelcoport, purchased from Supelco. The carrier gas was helium (30 mL/min flow), the detection was accomplished with flame ionization and monitored with HP-3390A reporting integrator. TLC was carried out using Merck pre-coated plates (60 F254, 250 μm).
2.2. 2-Methyl-7-(phenylsulfanylmethyl)naphthalene (3)
A mixture of 2-bromomethyl-7-methylnaphthalene (1) (0.200 g, 0.861 mmol), 2:1 (v/v) dichloromethane/acetonitrile (5 mL), 1M NaOH (5 mL), freshly distilled thiophenol (0.188 g, 1.71 mmol) and a drop of methyltricaprylammonium chloride (MTCAC) was vigorously stirred at 60 °C for 3 h under argon. The mixture was cooled to room temperature, the layers were separated and the organic layer was washed with 1M sodium hydroxide (2 × 10 mL), water (2 × 10 mL) and brine (1 × 10 mL). Drying over sodium sulfate and removal of the solvent in vacuo afforded a yellow solid. Recrystallization from ethanol yielded 0.121 g (54%) of 3 as white plates.
M.p. 89–91 °C.
Rf (20% dichloromethane in hexanes) = 0.26.
GC: Rt = 15.80 min (100 °C, 3 min, 8 °C/min to 280 °C).
1H NMR (400 MHz, CDCl3): δ = 7.72 (d, J = 8.4 Hz, 1 H), 7.69 (d, J = 8.4 Hz, 1 H), 7.58 (s, 1 H), 7.50 (s, 1 H), 7.38 (dd, J = 8.4 Hz, 1.7 Hz, 1 H), 7.34–7.29 (m, 2 H), 7.27 (dd, J = 8.4 Hz, 1.7 Hz, 1 H), 7.24–7.12 (m, 3 H), 4.24 (s, 2 H, Ar-CH2-SPh), 2.48 (s, 3 H, Ar-CH3).
13C NMR (100 MHz): δ = 136.35 (C), 135.78 (C), 134.87 (C), 133.52 (C), 130.82 (C), 129.90 (CH), 128.83 (CH), 128.08 (CH), 127.99 (CH), 127.43 (CH), 126.78 (CH), 126.68 (CH), 126.35 (CH), 126.08 (CH) (Ar), 39.41 (Ar-CH2-SPh), 21.70 (Ar-CH3).
DEPT-135 NMR: δ = 129.90 (↑, CH), 128.83 (↑, CH), 128.08 (↑, CH), 127.99 (↑, CH), 127.43 (↑, CH), 126.78 (↑, CH), 126.68 (↑, CH), 126.35 (↑, CH), 126.08 (↑, CH) (Ar), 39.41 (↓, Ar-CH2-SPh), 21.70 (↑, Ar-CH3).
IR (CCl4, cm-1): 3055, 3022, 2924, 2861, 1638, 1610, 1585, 1515, 1480, 1439, 1383, 1336, 1230, 1078, 1025, 1012, 959, 902.
EI-MS (m/z, rel. intensity): 265 (M+ + 1, 3%), 264 (M+, 7%), 197 (3%), 157 (9%), 156 (9%), 155 (M+ – SPh, 100%), 111 (14%).
3. Conclusion
The reaction of 2-bromomethyl-7-methylnaphthalene with thiophenol under phase-transfer catalytic conditions, in the presence of methyltricaprylammonium chloride (MTCAC) gave the desired product 3 in moderate yield. The structure of 3 was unambiguously determined by spectroscopic means (1H NMR, 13C NMR, DEPT-135 NMR, IR and MS). The reaction can be conveniently monitored by GC and/or TLC (20% dichloromethane in hexanes). The reaction was performed in degassed solution under argon atmosphere to avoid the oxidation of the thiophenol into diphenyl disulfide [9]. Diphenyl disulfide can pose a separation problem and can interfere with the mesolytic cleavage studies.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Maslak, P.; Narvaez, J.N. Mesolytic ceavage of C-C bonds. Comparison with homolytic and heterolytic processes in the same substrate. Angew. Chem. Int. Ed. Engl. 1990, 29, 283–285. [Google Scholar] [CrossRef]
- Frankel, M.; Gertner, D.; Jacobson, H.; Zilkha, A. Syntheses of Poly-S-Alkyl-L-Cysteines. J. Chem. Soc. 1960, 1390–1393. [Google Scholar] [CrossRef]
- Dymicky, M.; Byler, D.M. Synthesis of ethyl n-carbobenzoxytyrosyl-s-benzylcysteinylglycinate. Org. Prep. Proced. Int. 1991, 23, 93–101. [Google Scholar] [CrossRef]
- Vögtle, F.; Klieser, B. Cesium-ion assisted synthesis of strained compounds. Synthesis 1982, 294–296. [Google Scholar] [CrossRef]
- Yin, J.M.; Pidgeon, C. A simple and efficient method for preparation of unsymmetrical sulfides. Tetrahedron Lett. 1997, 38, 5953–5954. [Google Scholar] [CrossRef]
- Herriott, A.W.; Picker, D. Phase-transfer synthesis of sulfides and dithioacetals. Synthesis 1975, 447–448. [Google Scholar] [CrossRef]
- Maslak, P.; Guthrie, R.D. Electron apportionment in cleavage of radical-anions. 2. naphthylmethyl phenyl ethers vs. naphthyl benzyl ethers. J. Am. Chem. Soc. 1986, 108, 2637–2640. [Google Scholar] [CrossRef]
- Buuhoi, N.P.; Lecocq, J. Side-chain bromination of some alkylnaphthalenes with n-bromosuccinimide. J. Chem. Soc. 1946, 830–832. [Google Scholar] [CrossRef]
- McMurry, J. Organic Chemistry, 5th ed.; Brooks/Cole: Pacific Grove, CA, USA, 2000; p. 729. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).