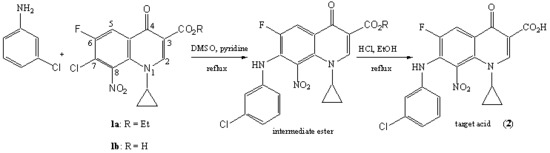
7-(3-Chlorophenylamino)-1-cyclopropyl-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Wise, R.; Andrews, J.M.; Edwards, L.J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob. Agents Chemother. 1983, 23, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Ennis, K.; Vercaigne, L.; Walkty, A.; Gin, A.S.; Embil, J.; Smith, H.; Hoban, D.J. A critical review of the fluoroquinolones: Focus on respiratory tract infections. Drugs 2002, 62, 13–59. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.D.; De Almeida, M.V.; De Souza, M.V.N.; Couri, M.R.C. Biological activity and synthetic metodologies for the preparation of fluoroquinolones, a class of potent antibacterial agents. Curr. Med. Chem. 2003, 10, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Al-Hiari, Y.; Al-Mazari, I.; Shakya, A.; Darwish, R.; Abu-Dahab, R. Synthesis and antibacterial properties of new 8-nitrofluoroquinolone derivatives. Molecules 2007, 12, 1240–1258. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.R.; Cai, S.; Dimitroff, M.; Fang, L.; Huh, K.K.; Ryckman, D.R.; Shang, X.; Shawar, R.M.; Therrien, J.H. A prodrug approach towards the development of water soluble fluoroquinolones and structure-activity relationships of quinoline-3-carboxylic acids. J. Med. Chem. 2004, 47, 4693–4709. [Google Scholar] [CrossRef] [PubMed]
- Ball, P. Quinolone generations: Natural history or natural selection. J. Antimicrobial. Chemother. 2000, 46, (Topic T1). 17–24. [Google Scholar] [CrossRef]
- Chen, F.J.; Lo, H.J. Molecular mechanisms of fluoroquinolone resistance. J. Microbiol. Immunol. Infect. 2003, 36, 1–9. [Google Scholar] [PubMed]
- Renau, T.E.; Sanchez, J.P.; Gage, J.W.; Dever, J.A.; Shapiro, M.A.; Grackeck, S.J.; Domagala, J.M. Structure-activity relationships of the quinolone antibacterials against mycobacteria: Effect of structural changes at N-1 and C-7. J. Med. Chem. 1996, 39, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Khalil, O.M.; Roshdy, S.M.A.; Shaaban, M.A.; Hasanein, M.K. New 7-substituted fluoroquinolones. Bull. Fac. Pharm. 2002, 40, 89–96. [Google Scholar]

© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Hiari, Y.M.; Qandil, A.M.; Al-Zoubi, R.M.; Alzweiri, M.H.; Darwish, R.M.; Shattat, G.F.; Al-Qirim, T.M. 7-(3-Chlorophenylamino)-1-cyclopropyl-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid. Molbank 2010, 2010, M669. https://doi.org/10.3390/M669
Al-Hiari YM, Qandil AM, Al-Zoubi RM, Alzweiri MH, Darwish RM, Shattat GF, Al-Qirim TM. 7-(3-Chlorophenylamino)-1-cyclopropyl-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid. Molbank. 2010; 2010(2):M669. https://doi.org/10.3390/M669
Chicago/Turabian StyleAl-Hiari, Yusuf Mohammad, Amjad M. Qandil, Rufaida M. Al-Zoubi, Muhammed H. Alzweiri, Rula M. Darwish, Ghassan F. Shattat, and Tariq M. Al-Qirim. 2010. "7-(3-Chlorophenylamino)-1-cyclopropyl-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid" Molbank 2010, no. 2: M669. https://doi.org/10.3390/M669
APA StyleAl-Hiari, Y. M., Qandil, A. M., Al-Zoubi, R. M., Alzweiri, M. H., Darwish, R. M., Shattat, G. F., & Al-Qirim, T. M. (2010). 7-(3-Chlorophenylamino)-1-cyclopropyl-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid. Molbank, 2010(2), M669. https://doi.org/10.3390/M669