(Benzoylamino)methyl 4-Hydroxybenzoate
Abstract
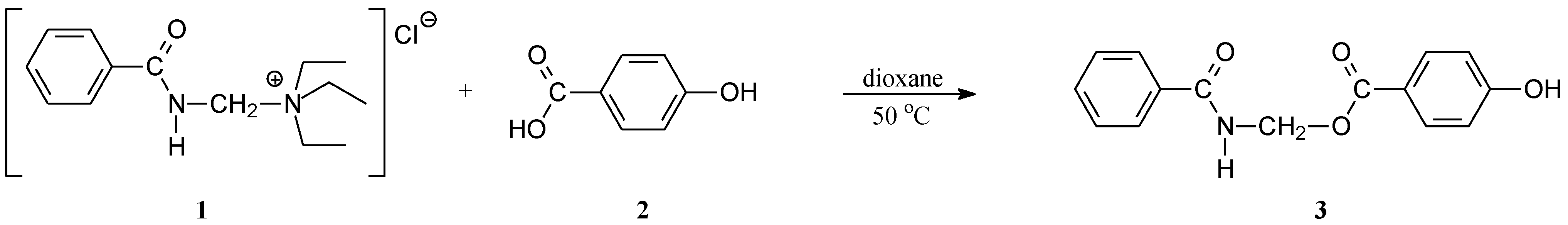
:
Experimental
(Benzoylamino)methyl 4-hydroxybenzoate (“Benzamidomethylparaben”) (3)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Andersen, F.A. Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int. J. Toxicol. 2008, 27 (Suppl. 4), 1–82. [Google Scholar]
- Lang, M.; Rye, R.M. Correlation between the antibacterial activity of some p-hydroxybenzoate esters and their cellular uptake. Microbios 1973, 7, 199–207. [Google Scholar] [PubMed]
- Bubnoff, M.V.; Schnell, D.; Vogt-Moykoff, J. Concerning the pharmacology of benzoic acid, p-chlorobenzoic acid, as well as p-hydroxybenzoic acid and its esters. Arzneim.-Forsch. 1957, 7, 340–344. [Google Scholar]
- Kitamura, Y. Effects of local anesthetics on the peripheral nerve and the spinal cord. Osaka City Med. J. 1979, 25, 7–24. [Google Scholar] [PubMed]
- Soni, M.G.; Carabin, I.G.; Burdock, G.A. Safety assessment of esters of p-hydroxybenzoic acid (paraben). Food Chem. Toxicol. 2005, 43, 985–1015. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; de Catanzaro, D. Estrogenicity of parabens revisited: Impact of parabens on early pregnancy and an uterotrophic assay in mice. Reprod. Toxicol. 2009, 28, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D.; Aljarrah, A.; Miller, W.R.; Coldham, N.G.; Sauer, M.J.; Pope, G.S. Concentrations of parabens in human breast tumours. J. Appl. Toxicol. 2004, 24, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D.; Harvey, P.W. Paraben esters: Review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J. Appl. Toxicol. 2008, 28, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.W.; Everett, D.J. Regulation of endocrine-disrupting chemicals: Critical overview and deficiencies in toxicology and risk assessment for human health. Best Pract. Res. Clin. Endoc. Met. 2006, 20, 145–165. [Google Scholar] [CrossRef] [PubMed]
- Golden, R.; Gandy, J.; Vollmer, G. A review of the endocrine activity of parabens and implications for potential risks to human health. Crit. Rev. Toxicol. 2005, 35, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Soni, M.G.; Taylor, S.L.; Greenberg, N.A.; Burdock, G.A. Evaluation of the health aspects of methyl paraben: A review of the published literature. Food Chem. Toxicol. 2002, 40, 1335–1373. [Google Scholar] [CrossRef]
- Chen, J.; Ahn, K.C.; Gee, N.A.; Gee, S.J.; Hammock, B.D.; Lasley, B.L. Antiandrogenic properties of parabens and other phenolic containing small molecules in personal care products. Toxicol. Appl. Pharmacol. 2007, 221, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.S.; Martins, F.C.; Oliveira, P.J.; Ramalho-Santos, J.; Peixoto, F.P. Parabens in male infertility—Is there a mitochondrial connection? Reprod. Toxicol. 2009, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Oishi, S. Effects of butylparaben on the male reproductive system in rats. Toxicol. Ind. Health 2001, 17, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Oishi, S. Effects of propylparaben on the male reproductive system. Food Chem. Toxicol. 2002, 40, 1807–1813. [Google Scholar] [CrossRef]
- Maggi, L.; Carmona, M.; Zalacain, A.; Tomé, М.M.; Murcia, M.A.; Alonso, G.L. Parabens as agents for improving crocetin esters’ shelf-life in aqueous saffron extracts. Molecules 2009, 14, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Asnani, V.M.; Verma, R.J. Ameliorative effects of ginger extract on paraben-induced lipid peroxidation in the liver of mice. Acta Pol. Pharm.-Drug Res. 2009, 66, 225–228. [Google Scholar]
- Vo, T.T.B.; Jeung, E. An evaluation of estrogenic activity of parabens using uterine calbindin-D9k gene in an immature rat model. Toxicol. Sci. 2009, 112, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, M.K.; Parajuli, R.; Phukan, P. Synthesis of parabens using montmorillonite K10 clay as Catalyst: A green protocol. Indian J. Chem. Technol. 2007, 14, 104–106. [Google Scholar]
- Quévrain, E.; Domart-Coulon, I.; Pernice, M.; Bourguet-Kondracki, M. Novel natural parabens produced by a Microbulbifer bacterium in its calcareous sponge host Leuconia nivea. Environ. Microbiol. 2009, 11, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, K.A.; Singh, K.V.; Bajpai, A.; Shukla, G.; Singh, S.; Mishra, K.A. Synthesis and biological properties of 4-(3H)-quinazolone derivatives. Eur. J. Med. Chem. 2007, 42, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Zlotin, G.S.; Sharova, V.I.; Luk`yanov, A.O. Chemical properties of N-(amidomethyl)- and N-(imidomethyl)glycine derivatives 2. Reactions at alkoxycarbonyl and carboxyl groups. Rus. Chem. Bul. 1996, 45, 1680–1687. [Google Scholar] [CrossRef]
- Csomós, P.; Fodor, L.; Bernáth, G.; Csámpai, A.; Sohár, P. Synthesis of 4-thiaharmalan analogue 4-aryl-1,3-thiazino[5,6-b]indole derivatives by prevention of rearrangements to position two of the indole moiety. Tetrahedron 2008, 64, 8646–8651. [Google Scholar] [CrossRef]
- Csomós, P.; Fodor, L.; Bernáth, G.; Csámpai, A.; Sohár, P. An expeditious synthesis for g-carboline analogue 4-aryl-1,3-thiazino[6,5-b]indole derivatives via the trifluoromethanesulfonic acid-promoted isomerization of 3-amidomethylthioindole intermediates to 2-indolyl sulphides. Tetrahedron 2009, 65, 1475–1480. [Google Scholar] [CrossRef]
- Mai, A.; Artico, M.; Valente, S.; Cerbara, I.; Befani, O.; Turini, P.; Vedova, D.L.; Agostinelli, E. Synthesis and biochemical evaluation of (R)-5-acyloxymethyl- and (S)-5-acylaminomethyl-3-(1H-pyrrol-1-yl)-2-oxazolidinones as new anti-monoamine oxidase (anti-MAO) agents. ARKIVOC 2004, v, 32–43. [Google Scholar]
- Noyori, R.; Kitamura, M.; Ohkuma, T. Toward efficient asymmetric hydrogenation: Architectural and functional engineering of chiral molecular catalysts. PNAS 2004, 101, 5356–5362. [Google Scholar] [CrossRef] [PubMed]
- Cesarotti, E.; Rimoldi, I.; Spalluto, P.; Demartin, F. Chiral 1,4-bis-diphosphine ligands from optically active (Z)-olefines. Tetrahedron-Asymmetr. 2007, 18, 1278–1283. [Google Scholar] [CrossRef]
- Mateska, A.; Stojković, G.; Mikhova, B.; Mladenovska, K.; Popovski, E. Carbon-carbon bond formation in aqueous media. Benzamidomethylation of some carbon nucleophiles. ARKIVOC 2009, 131–140. [Google Scholar]
- Shimoda, K.; Kubota, N.; Hamada, H.; Kobayashi, T.; Hamada, H.; Shafi, S.M.; Nakajima, N. Production of (2R,3S)-2-benzamidomethyl-3-hydroxybutanoates by immobilized plant cells of parthenocissus tricuspidata. Biochemistry Insights 2009, 2, 5–7. [Google Scholar]
- Shimoda, K.; Kubota, N.; Hamada, H.; Hamada, H. Diastereoselective reduction of β-keto carbonyl compounds by cultured plant cells. Tetrahedron Lett. 2006, 47, 1541–1544. [Google Scholar] [CrossRef]
- Kumobayashi, H.; Miura, T.; Sayo, N.; Saito, T. The development of the new processes for manufacturing the key intermediates of β-lactam antibiotics. J. Syn. Org. Chem. Jpn. 1999, 57, 387–393. [Google Scholar] [CrossRef]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Simple method for benzamidomethylation of phenols in water solution. Syn. Commun. 1999, 29, 3451–3458. [Google Scholar] [CrossRef]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Benzamidomethylation with (benzamidomethyl)triethylammonium chloride 2. A simple method for benzamidomethylation of thiols, amines and carboxylic acids. Molecules 2000, 5, 927–936. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Popovski, E.; Mladenovska, K. (Benzoylamino)methyl 4-Hydroxybenzoate. Molbank 2010, 2010, M658. https://doi.org/10.3390/M658
Popovski E, Mladenovska K. (Benzoylamino)methyl 4-Hydroxybenzoate. Molbank. 2010; 2010(1):M658. https://doi.org/10.3390/M658
Chicago/Turabian StylePopovski, Emil, and Kristina Mladenovska. 2010. "(Benzoylamino)methyl 4-Hydroxybenzoate" Molbank 2010, no. 1: M658. https://doi.org/10.3390/M658
APA StylePopovski, E., & Mladenovska, K. (2010). (Benzoylamino)methyl 4-Hydroxybenzoate. Molbank, 2010(1), M658. https://doi.org/10.3390/M658



