N-[1-(2,5-Dimethyl-3-thienyl)ethylidene]-1,3-benzothiazol-2-amine
Abstract
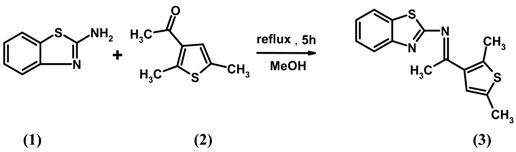
:
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Przybylski, P.; Pyta, K.; Stefanska, J.; Ratajczak-Sitarz, M.; Katrusiak, A.; Huczynski, A.; Brzezinski, B. Synthesis, crystal structures and antibacterial activity studies of aza-derivatives of phytoalexin from cotton plant–gossypol. Eur. J. Med. Chem. 2009, 44, 4393–4403. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, R.; Maheswaran, S. Synthesis, spectra, dioxygen affinity and antifungal activity of Ru(III) Schiff base complexes. J. Inorg. Biochem. 2003, 96, 457–462. [Google Scholar] [CrossRef]
- Silveira, V.C.; Luz, J.S.; Oliveira, C.C.; Graziani, I.; Ciriolo, M.R.; Ferreira, A.M.C. Double-strand DNA cleavage induced by oxindole-Schiff base copper(II) complexes with potential antitumor activity. J. Inorg. Biochem. 2008, 102, 1090–1103. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, S.N.; Sriram, D.; Nath, G.; Clercq, E. Synthesis, antibacterial, antifungal and anti-HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(4′-chlorophenyl)thiazol-2-yl]thiosemicarbazide. Eur. J. Pharma. Scie. 1999, 9, 25–31. [Google Scholar] [CrossRef]
- Holla, B.S.; Malini, K.V.; Rao, V.S.; Sarojini, B.K.; Kumari, N.S. Synthesis of some new 2,4-disubstituted thiazoles as possible antibacterial and anti-inflammatory agents. Eur. J. Med. Chem. 2003, 38, 313–318. [Google Scholar] [CrossRef]
- El-Sabbagh, O.I.; Baraka, M.M.; Ibrahim, S.M.; Pannecouque, C.; Graciela, Andrei; Snoeck, R.; Balzarini, J.; Rashad, A.A. Synthesis and antiviral activity of new pyrazole and thiazole derivatives. Eur. J. Med. Chem. 2009, 44, 3746–3753. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjuna, B.P.; Sastry, B.S.; Kumar, G.V.S.; Rajendraprasad, Y.; Chandrashekar, S.M.; Sathisha, K. Synthesis of new 4-isopropylthiazole hydrazide analogs and some derived clubbed triazole, oxadiazole ring systems–A novel class of potential antibacterial, antifungal and antitubercular agents. Eur. J. Med. Chem. 2009, 44, 4739–4746. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Asiri, A.M.; Khan, S.A. N-[1-(2,5-Dimethyl-3-thienyl)ethylidene]-1,3-benzothiazol-2-amine. Molbank 2010, 2010, M659. https://doi.org/10.3390/M659
Asiri AM, Khan SA. N-[1-(2,5-Dimethyl-3-thienyl)ethylidene]-1,3-benzothiazol-2-amine. Molbank. 2010; 2010(1):M659. https://doi.org/10.3390/M659
Chicago/Turabian StyleAsiri, Abdullah Mohamed, and Salman A. Khan. 2010. "N-[1-(2,5-Dimethyl-3-thienyl)ethylidene]-1,3-benzothiazol-2-amine" Molbank 2010, no. 1: M659. https://doi.org/10.3390/M659
APA StyleAsiri, A. M., & Khan, S. A. (2010). N-[1-(2,5-Dimethyl-3-thienyl)ethylidene]-1,3-benzothiazol-2-amine. Molbank, 2010(1), M659. https://doi.org/10.3390/M659




