Abstract
The N-ethoxycarbonylhexyl quaternary salt of lepidine has been synthesized in one step in 56% yield. 1H and 13C NMR data are reported.
Introduction
N-alkyl quaternary ammonium salts are used extensively as precursors of photochromic spiropyrans [1], RNA antagonists [2], and various cyanine dyes [3]. They are usually prepared from the reaction between a heterocyclic base and an alkyl halide refluxing in solvent for 6–48 h [4]. One example requires refluxing in acetonitrile for 24 h, treatment with diethyl ether followed by filtration [3]. This process was repeated 1-3 times on the concentrated filtrates to achieve the published yields. An interesting RNA antagonist precursor, 1-(6-ethoxy-6-oxohexyl)-4-methylquinolinium iodide quaternary ammonium salt (1a), has been synthesized, but the experimental details and characterization of the compound was not reported [2]. However, 1-(6-methoxy-6-oxohexyl)-4-methylquinolinium chloride quaternary salt 1b has been synthesized in two steps in 37% overall yield (Scheme 1) [5]. We report the rapid solvent-free synthesis of the N-ethoxycarbonylhexyl quaternary salt (1a) from commercially available lepidine in one step in 56% yield with minimal purification.
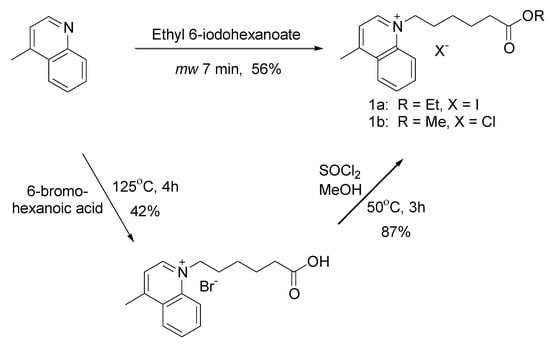
Scheme 1.
Synthesis of quaternary salt.
Experimental Section
General
1H and 13C NMR spectra were obtained using a Varian Gemini 400 NMR and were recorded at 400 MHz and 100 MHz respectively. High resolution ESI-MS was acquired with a Bruker Apex-Qe instrument. All reagents and chemicals were obtained from Aldrich Chemical Company (USA) and were used as received. Microwave reactions conducted in a Biotage Initiaor ESP.
Synthesis of 1-(6-ethoxy-6-oxohexyl)-4-methylquinolinium iodide
Lepidine (0.250 g, 1.754 mmol) and ethyl 6-iodohexanoate (0.522 g, 1.929 mmol) were combined in a 5 mL Biotage microwave vial equipped with a stir bar. The vial was placed in the Biotage microwave with a ramp time of 4 min and held at 120 °C for 5 min. The vial with reaction mixture was allowed to sit for 5 min. and irradiated again with a ramp time of 4 min and held at 120 oC for 2 min. The resulting dark brown solid was dissolved in a mixture of ethyl acetate (5 mL) and methanol (0.5 mL). The dark brown mixture was purified by eluting on a coarse frit packed with silica gel. Ethyl acetate (400 mL) was used to wash the product and a mixture of dichloromethane and methanol (90:10) was used for subsequent washes. The dichloromethane and methanol washes were concentrated and dried to obtain a brown oil (56%).
1H NMR (400 MHz, DMSO-d6) δ ppm: 9.45 (d, J = 6.05 Hz, 2H), 8.56 (m, 2H), 8.27 (m, 1H), 8.08 (m, 2H), 5.02 (t, J = 7.47 Hz, 2H), 4.02 (q, J = 7.11, Hz, 2H), 3.00 (s, 3H), 1.95 (m, 2H), 1.60 (m, 2H), 1.55 (m, 2H), 1.38 (m, 2H), 1.15 (t, J = 7.47 Hz, 3H).
13C NMR (100 MHz, DMSO-d6): 172.7 (C), 158.5 (C), 148.4 (CH), 136.6 (CH), 135.0 (CH), 129.5 (CH), 128.9 (CH), 127.1 (CH), 122.6 (CH), 119.3 (CH), 59.7 (CH2), 56.6 (CH2), 33.1 (CH2), 29.0 (CH2), 25.1, (CH2), 23.8 (CH2), 19.7 (CH3), 14.1 (CH3).
HR ESI-MS for C18H24NO2: m/z = 286.1805; calculated: 286.1807.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
This study was supported by the following grants: NSF 0236753, and NSF HRD-0627276.
References and Notes
- Hirano, M.; Osakada, K.; Nohira, H.; Miyashita, A. Crystal and solution structures of photochromic spirobenzothiopyran. First full characterization of the meta-stable colored species. J. Org. Chem. 2002, 67, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Tok, J.B.H.; Cho, J.; Rando, R.R. Aminoglycoside hybrids as potent RNA antagonists. Tetrahedron 1999, 55, 5741–5758. [Google Scholar] [CrossRef]
- Mirshra, A.; Behera, R.K.; Behera, B.K.; Mishra, B.K.; Behera, G.B. Cyanines during the 1990s: A review. Chem. Rev. 2000, 100, 1973–2011. [Google Scholar] [CrossRef]
- Pardal, A.C.; Ramos, S.S.; Santos, P.F.; Reis, L.V.; Almeida, P. Synthesis and spectroscopic characterisation of n-Alkyl quaternary ammonium salts typical precursors of cyanines. Molecules 2002, 7, 320–330. [Google Scholar] [CrossRef]
- Carreon, J.R.; Stewart, K.M.; Mahon, K.P., Jr.; Shin, S.; Kelley, S.O. Cyanine dye conjugates as probes for live cell imaging. Bio. Med. Chem. Lett. 2007, 17, 5182–5185. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).