Abstract
Dimethyl 1,4-dihydro-2,6-dimethyl-1-(4-methylphenyl)-4-(4-methoxylphenyl)–pyridine-3,5-dicarboxylate has been synthesized via Hantzsch condensation reaction of p-methoxybenzaldehyde, methyl acetoacetate and p-toluidine promoted by microwave irradiation (MWI) in the presence of iodine under solvent-free conditions.
Hantzsch 1,4-dihydropyridines (1,4-DHPs) are well known as Ca2+ channel blockers [1], and have emerged as one of the most important classes of drugs for the treatment of cardiovascular diseases [2] including hypertension [3]. Shah and co-workers have studied the role of 1,4-dihydropyridines as chemotherapeutic agents, such as multi-drug-resistance (mdr) reversal in tumor cells [4], potential immunomodulating [5] and antitubercular compounds as well [6,7]. In light of this, the further study of N-substituted DHP skeleton proved to be important for mdr reversal in tumor cells [8]. Thus, the synthesis of N-substituted 1,4-dihydropyridines is of considerable importance.
The most classical synthesis of symmetrical 1,4-DHPs is the three-component condensation of aryl aldehydes, ammonia, or amines and β-ketoesters, which was reported in 1882 by Hantzsch [9]. However, the yields of 1,4-DHPs obtained by the Hantzsch method are generally low. Modified synthetic methodologies [10,11,12,13,14] improved the yields but using expensive reagents and require longer reaction times. Thus, the development of an efficient and versatile method for the preparation of Hantzsch 1,4-DHPs is an active ongoing research area. The eco-friendly goal of making organic compounds without using solvents has come several steps closer in recent years [15]. And the microwave activation stands among the alternative routes proposed during the past decade due to the drastic reduction of reaction times [16,17,18]. Recently, the synthesis of organic compounds assisted by microwaves [19] under solvent free conditions [20,21], is an improved technique. Due to greater selectivity, rapid transfer of energy, significant practical simplicity and pure products, microwave-assisted reactions have greater advantages over conventional homogeneous methods. In recent times, the use of molecular iodine [22,23,24,25] has received considerable attention as an inexpensive, nontoxic, readily available catalyst for various organic transformations to afford the corresponding products in excellent yields with high selectivity. Owing to numerous advantages associated with this eco-friendly element, iodine has been explored as a good catalyst for various organic synthesis [26,27,28].
Herein, we report the synthesis of dimethyl 1,4-dihydro-2,6-dimethyl-1-(4-methylphenyl)-4-(4-methoxylphenyl)pyridine-3,5-dicarboxylate 1 via Hantzsch condensation reaction of p-methoxy-benzaldehyde, methyl acetoacetate and p-toluidine promoted by microwave irradiation (MWI) in the presence of iodine under solvent-free conditions (Scheme 1). The title compound has been fully characterized by NMR (1H and 13C), IR, MS, and elemental analysis. This protocol is proven to be efficient and environmentally benign.
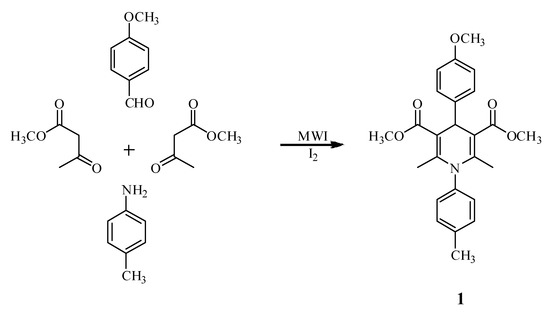
Scheme 1.
Experimental Procedure
A mixture of p-methoxybenzaldehyde (0.68 g, 5 mmol), methyl acetoacetate (1.39 g, 12 mmol), p-toluidime (0.54 g, 5 mmol) with a catalytic amount of iodine was irradiated in a microwave reactor (800 W) for 15 min at 80 °C. After completion of the reaction as indicated by TLC, the crude product was purified by flash column chromatography on silica gel (300-400 mesh) and eluted with ethyl acetate-petroleum ether (1:12) to afford the title compound 1 as white crystals, 1.75 g, yield 83.2%, m.p. 153~155 °C.
Moreover, our investigation showed that the best results were obtained when the molar ratio of aldehyde, acetoacetate and p-toluidime was 1:2.4:1. In addition, the best yield is achieved when the amount of iodine is 3% based on the substrate.
Structural Characterization
1H NMR (Bruker 300 MHz, CDCl3): δH 7.26 (d, J = 8.4 Hz, 2 H, Ar-H), 7.22 (d, J = 7.8 Hz, 2H, Ar-H), 6.98 (d, J = 7.8 Hz, 2 H, Ar-H), 6.83 (d, J = 8.4 Hz, 2 H, Ar-H), 5.07 (s, 1 H, CH), 3.78 (s, 3 H, OCH3), 3.68 (s, 6 H, 2*OCH3), 2.40 (s, 3 H, PhCH3), 2.05 (s, 6 H, 2*CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 168.56, 158.06, 147.67, 139.27, 138.66, 137.71, 129.95, 128.17, 113.57, 105.67, 55.14, 51.14, 37.59, 21.09, 18.55 ppm. IR (Bruker Tensor 27, KBr): 3392, 2951, 1677, 727 cm–1. MS (AGILENT 5973N MSD, EI): m/z 422.6 (M++1). Elemental Anal. (Perkin Elmer PE 2400 II HONS): calcd for C25H27NO5 (421.49): C, 71.24; H, 6.46; N, 3.32; O, 18.98. Found: C, 71.3; H, 6.3; N, 3.4; O, 18.8.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The authors are grateful to Zhixian Liu and Lixin Yu for determinations of NMR, IR and elemental analysis.
References
- Bocker, R.H.; Guengerich, F.P. Oxidation of 4-aryl- and 4-alkyl-substituted 2,6-dimethyl-3,5-bis(alkoxycarbonyl)-1,4-dihydropyridines by human liver microsomes and immunochemical evidence for the involvement of a form of cytochrome P-450. J. Med. Chem. 1986, 29, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Bossert, F.; Meyer, H.; Wehinger, E. 4-Aryldihydropyridines, a new class of highly active calcium antagonists. Angew. Chem. Int. Ed. Engl. 1981, 20, 762–769. [Google Scholar] [CrossRef]
- Nakayama, H.; Kasoaka, Y. Chemical identification of binding sites for calcium channel antagonists. Heterocycles 1996, 42, 901–909. [Google Scholar] [CrossRef]
- Smith, W.T., Jr.; Kort, P.G. The synthesis of substituted β-arylglutaric acids. J. Am. Chem. Soc. 1950, 72, 1877–1878. [Google Scholar] [CrossRef]
- Michal, F.; Moller, W. Pyridin-Synthesen, II. Pyridin-derivate aus 2.3.4.5.6-pentaacetyl-al-d-glucose. Justus Liebigs Ann. Chem. 1963, 670, 63–68. [Google Scholar] [CrossRef]
- Shah, A.; Gevariya, H.; Motohashi, N.; Kawase, M.; Saito, S.; Sakagami, H.; Satoh, Y.; Solymosi, A.; Walfard, K.; Molnar, J. 3,5-Diacetyl-1,4-dihydropyridines: Synthesis and MDR reversal in tumor cells. Anticancer Res. 2000, 20, 373–377. [Google Scholar] [PubMed]
- Shah, A.; Gevariya, H.; Motohashi, N.; Kawase, M.; Farkas, S.; Gyorgyi, G.; Molnar, J. Interaction between 3,5-diacetyl-1,4-dihydropyridines and ampicillin, and erythromycin on different E-coli strains. Int. J. Antimicrobial Agents 2002, 20, 227–229. [Google Scholar]
- Desai, B.; Sureja, D.; Naliapara, Y.; Saxena, A.K.; Shah, A. Synthesis and QSAR studies of 4-substituted phenyl-2,6-dimethyl-3,5-bis-N-(substituted phenyl)carbamoyl-1,4-dihydropyridines as potential antitubercular agents. Bioorg. Med. Chem. 2001, 9, 1993–1998. [Google Scholar] [CrossRef]
- Hantzsch, A. Condensationprodukte aus Aldehydammoniak und Ketoniartigen Verbindungen. Chem. Ber. 1881, 14, 1637–1638. [Google Scholar] [CrossRef]
- Lachowicz, B. Solid-phase synthesis of 4-aryl-1,4-dihydropyridines via the Hantzsch three component condensation. Monatsh. Chem. 1896, 17, 343. [Google Scholar] [CrossRef]
- Breitenbucher, J.G.; Figliozzi, G. Solid-phase synthesis of 4-aryl-1,4-dihydropyridines via the Hantzsch three component condensation. Tetrahedron Lett. 2000, 41, 4311–4315. [Google Scholar] [CrossRef]
- Anderson, A.G.; Berkelhammer, G. A Study of the Primary Acid Reaction on Model Compounds of Reduced Diphosphopyridine Nucleotide1,2. J. Am. Chem. Soc. 1958, 80, 992–999. [Google Scholar] [CrossRef]
- Erickson, J.G. The reactions of aldehydes with β-anilinocrotonic esters1. J. Am. Chem. Soc. 1945, 67, 1382–1386. [Google Scholar] [CrossRef]
- Maquestiau, A.; Maeyence, A.; Eynde, J.J.V. Ultrasound-promoted aromatization of hantzsch 1,4-Dihydropyridines by clay-supported cuptic nitrate. Tetrahedron Lett. 1991, 32, 3839–3840. [Google Scholar] [CrossRef]
- Dittmer, D.C. ‘No-solvent’ organic synthesis. (organic compounds synthesis without solvents). Chem. Ind. (London) 1997, 19, 779. [Google Scholar]
- Anniyappan, M.; Muralidhran, D.; Perumal, P.T. Synthesis of Hantzsch 1,4-dihydropyridines under microwave irradiation. Synth. Commun. 2002, 32, 659–663. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Reddy, P.T. Unprecedented synthesis of hantzsch 1,4-dihydropyridines under biginelli reaction conditions. Synth. Commun. 2001, 31, 425–430. [Google Scholar] [CrossRef]
- Cotterill, I.C.; Usyatinsky, A.Y.; Arnold, J.M.; Clark, D.S.; Dordick, J.S.; Michels, P.C.; Khmelnitsky, Y.L. Microwave assisted combinatorial chemistry synthesis of substituted pyridines. Tetrahedron Lett. 1998, 39, 1117–1120. [Google Scholar] [CrossRef]
- Perreuex, L.; Loupy, A. A tentative rationalization of microwave effects in organic synthesis according to the reaction medium, and mechanistic considerations. Tetrahedron 2001, 57, 9199–9223. [Google Scholar] [CrossRef]
- Varma, R.S.; Chatterjee, A.K.; Varma, M. Alumina-mediated microwave thermolysis: A new approach to deprotection of benzyl esters. Tetrahedron Lett. 1993, 34, 4603–4606. [Google Scholar] [CrossRef]
- Selvi, S.T.; Nadaraj, V.; Mohan, S.; Sasi, R.; Hema, M. Solvent free microwave synthesis and evaluation of antimicrobial activity of pyrimido[4,5-b]- and pyrazolo[3,4-b]quinolines. Bioorg. Med. Chem. 2006, 14, 3896–3903. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Ryu, E.K. Unusual iodine catalyzed lactonization of gamma-methyl-gamma,delta-pentenoic acids: A facile synthesis of gamma,gamma-dimethyl-gamma-butyrolactones. Tetrahedron Lett. 1996, 37, 1441–1444. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Hazarkhani, H. Iodine catalyzes efficient and chemoselective thioacetalization of carbonyl functions, transthioacetalization of O,O- and S,O-acetals and acylals. J. Org. Chem. 2001, 66, 7527–7529. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, H.; Iranpoor, N.; Sobhani, S. A high yielding preparation of alpha-trimethylsilyloxyphosphonates by silylation of alpha-hydroxyphosphonates with HMDS catalyzed by iodine. Tetrahedron Lett. 2002, 43, 3653–3655. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Shaikh, K.A. Molecular iodine-catalyzed efficient and highly rapid synthesis of bis(indolyl)methanes under mild conditions. Tetrahedron Lett. 2003, 44, 1959–1961. [Google Scholar] [CrossRef]
- Banik, B.K.; Mukhopadhyay, C.; Venkatraman, M.S.; Becker, F.F. A facile reduction of aromatic nitro compounds to aromatic amines by samarium and iodine. Tetrahedron Lett. 1998, 39, 7243–7246. [Google Scholar] [CrossRef]
- Banik, B.K.; Zegrocka, O.; Banik, I.; Hackfeld, L.; Becker, F.F. Samarium-induced iodine-catalyzed reduction of imines: synthesis of amine derivatives. Tetrahedron Lett. 1999, 40, 6731–6734. [Google Scholar] [CrossRef]
- Ko, S.; Sastry, M.N.V.; Lin, C.; Yao, C.F. Molecular iodine-catalyzed one-pot synthesis of 4-substituted-1,4-dihydropyridine derivatives via Hantzsch reaction. Tetrahedron Lett. 2005, 46, 5771–5774. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).