Synthesis of a 2-Furylpyrazoline Derivative Using Microwave Irradiation
Abstract
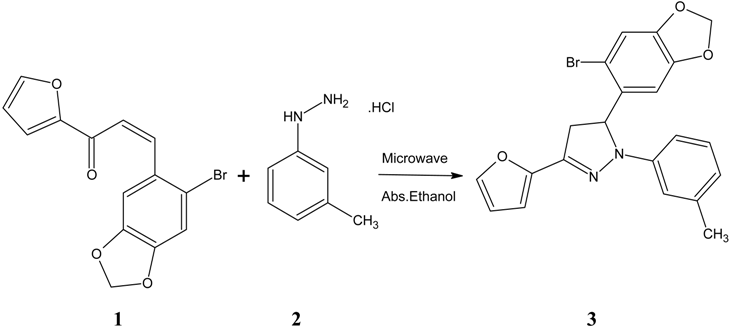
:
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References and Notes
- Özdemir, Z.; Kandilci, H.B.; Gümüsel, B.; Calıs, U.; Bilgin, A.A. Synthesis and studies on antidepressant and anticonvulsant activities of some 3-(2-furyl)pyrazoline derivatives. Eur. J. Med. Chem. 2007, 42, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Holla, B.S.; Akberali, P.M.; Shivananda, M.K. Studies on arylfuran derivatives. Part X. Synthesis and antibacterial properties of arylfuryl-2-pyrazolines. Il Farmaco 2000, 55, 256–263. [Google Scholar] [PubMed]
- Khan, S.S.; Hasan, A. Synthesis of some new bioactive 1-N-substituted 3,5-diaryl-2-pyrazolines. Heterocyclic Commun. 2007, 13, 131–138. [Google Scholar] [CrossRef]
- Kidwai, M.; Kukreja, S.; Thakur, R. K2CO3-mediated regioselective synthesis of isoxazoles and pyrazolines. Lett. Org. Chem. 2006, 3, 135–139. [Google Scholar] [CrossRef]
- Abdel-Magid; Ahmed, F.; Koskinen. Barium Hydroxide. In e-EROS Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd: USA, 2001. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jothikrishnan, B.T.; Shafi, S.S. Synthesis of a 2-Furylpyrazoline Derivative Using Microwave Irradiation. Molbank 2009, 2009, M613. https://doi.org/10.3390/M613
Jothikrishnan BT, Shafi SS. Synthesis of a 2-Furylpyrazoline Derivative Using Microwave Irradiation. Molbank. 2009; 2009(3):M613. https://doi.org/10.3390/M613
Chicago/Turabian StyleJothikrishnan, Balapragalathan Thappali, and Suban Syed Shafi. 2009. "Synthesis of a 2-Furylpyrazoline Derivative Using Microwave Irradiation" Molbank 2009, no. 3: M613. https://doi.org/10.3390/M613
APA StyleJothikrishnan, B. T., & Shafi, S. S. (2009). Synthesis of a 2-Furylpyrazoline Derivative Using Microwave Irradiation. Molbank, 2009(3), M613. https://doi.org/10.3390/M613



