2-(4-Pentyloxyphenyl)benzothiazole
Abstract
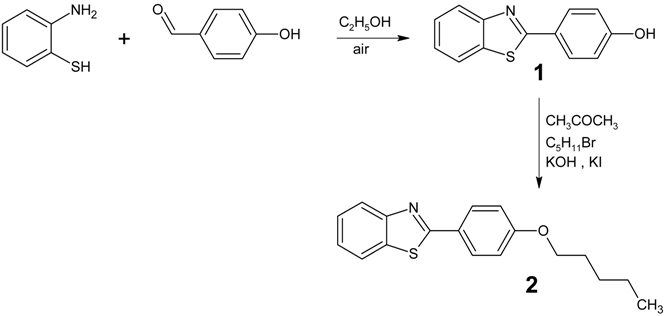
:
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References and Notes
- Chen, X.L.; Jenekhe, A. Macromolecules 1996, 29, 6189–6192.
- Chou, S.S.P.; Sun, D.J.; Lin, H.C.; Yang, P.K. Chem. Commun. 1996, 1045–1046.
- Raimundo, J.M.; Blanchard, P.; Ledoux-Rax, I.; Hierle, R.; Michaux, L.; Roncali, J. Chem. Commun. 2000, 1597–1598.
- Lee, C.H.; Yamamoto, T. Mol. Cryst. Liq. Cryst. 2001, 363, 77–84.
- Maruyama, T.; Suganuma, H.; Yamamoto, T. Synthetic Metals 1995, 74, 183–185.
- Prajapati, A. K.; Bonde, N. L. J. Chem. Sci. 2006, 118, 203.
- Ha, S.T.; Koh, T.M.; Yeap, G.Y.; Lin, H.C.; Boey, P.L.; Yip, F.W.; Ong, S.T.; Ong, L.K. Mol. Cryst. Liq. Cryst. 2009, 506, 56.
- Ha, S.T.; Koh, T.M.; Yeap, G.Y.; Lin, H.C.; Beh, J.K.; Win, Y.F.; Boey, P.L. Chin. Chem. Lett. 2009, 20, 1081.
- Bogert, M.T.; Corbitt, H.B. J. Am. Chem. Soc. 1926, 48, 783.
- Ha, S.T.; Ong, L.K.; Wong, J.P.W.; Win, Y.F.; Koh, T.M. Molbank 2009, 1, M598.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ha, S.T.; Koh, T.M.; Ong, S.T.; Lee, T.L.; Sivasothy, Y. 2-(4-Pentyloxyphenyl)benzothiazole. Molbank 2009, 2009, M611. https://doi.org/10.3390/M611
Ha ST, Koh TM, Ong ST, Lee TL, Sivasothy Y. 2-(4-Pentyloxyphenyl)benzothiazole. Molbank. 2009; 2009(3):M611. https://doi.org/10.3390/M611
Chicago/Turabian StyleHa, Sie Tiong, Teck Ming Koh, Siew Teng Ong, Teck Leong Lee, and Yasodha Sivasothy. 2009. "2-(4-Pentyloxyphenyl)benzothiazole" Molbank 2009, no. 3: M611. https://doi.org/10.3390/M611
APA StyleHa, S. T., Koh, T. M., Ong, S. T., Lee, T. L., & Sivasothy, Y. (2009). 2-(4-Pentyloxyphenyl)benzothiazole. Molbank, 2009(3), M611. https://doi.org/10.3390/M611



