Synthesis of 2-[4-(4-Chlorophenyl)piperazin-1-yl]-2-methylpropanoic Acid Ethyl Ester
Abstract
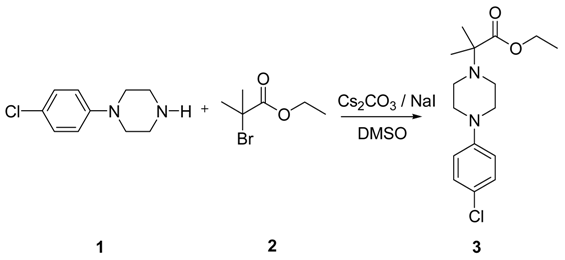
:
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Pati, H.N.; Das, U.; Clercq, E.D.; Balzarini, J.; Dimmock, J.R. Molecular modifications of 2-arylidene-1-indanones leading to increased cytotoxic potencies. J. Enz. Inhib. Med. Chem. 2007, 22, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Pati, H.N.; Das, U.; Ramirez-Erosa, I.J.; Dunlop, D.M.; Hickie, R.A.; Dimmock, J.R. α,β-Substituted 1-aryl-3-dimethylaminopropanone hydrochlorides: potent cytotoxins towards human WiDr colon cancer cells. Chem. Pharm. Bull. 2007, 55, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Pati, H.N.; Das, U.; Das, S.; Bandy, B.; Clercq, E.D.; Balzarini, J.; Kawase, M.; Sakagami, H.; Quail, J.W.; Stables, J.P.; Dimmock, J.R. The cytotoxic properties and preferential toxicity to tumor cells displayed by some 2,4-bis(benzylidene)- 8- methyl-8-azabicyclo[3.2.1]octan-3-ones and 3,5-bis(benzylidene)-1-methyl-4-piperidones. Euro. J. Med. Chem. 2009, 44, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Pati, H.N.; Das, U.; Sharma, R.K.; Dimmock, J.R. Cytotoxic thiol alkylators. Mini-Rev. Med. Chem. 2007, 7, 131–139. [Google Scholar] [CrossRef] [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pati, H.N.; Mishra, B.K.; Satam, V.S. Synthesis of 2-[4-(4-Chlorophenyl)piperazin-1-yl]-2-methylpropanoic Acid Ethyl Ester. Molbank 2009, 2009, M607. https://doi.org/10.3390/M607
Pati HN, Mishra BK, Satam VS. Synthesis of 2-[4-(4-Chlorophenyl)piperazin-1-yl]-2-methylpropanoic Acid Ethyl Ester. Molbank. 2009; 2009(3):M607. https://doi.org/10.3390/M607
Chicago/Turabian StylePati, Hari N., Bijay K. Mishra, and Vijay S. Satam. 2009. "Synthesis of 2-[4-(4-Chlorophenyl)piperazin-1-yl]-2-methylpropanoic Acid Ethyl Ester" Molbank 2009, no. 3: M607. https://doi.org/10.3390/M607
APA StylePati, H. N., Mishra, B. K., & Satam, V. S. (2009). Synthesis of 2-[4-(4-Chlorophenyl)piperazin-1-yl]-2-methylpropanoic Acid Ethyl Ester. Molbank, 2009(3), M607. https://doi.org/10.3390/M607



