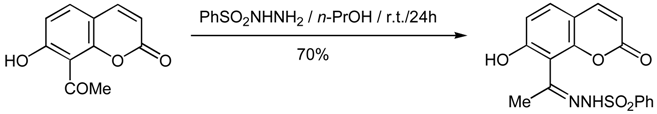
7-Hydroxy-8-acetylcoumarin N-Phenylsulfonylhydrazone
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Kotali, A.; Harris, P. A. Org. Prep. Proc. Int. 1994, 26(2), 155.
- Kostova, I. Curr. Med. Chem.-Anti Cancer Agents 2005, 5, 29.
- Cerecetto, H.; Porcal, W. Mini-Rev. in Med. Cmem. 2005, 5, 57.
- Abramov, M. A.; Dehaen, W. Synthesis 2000, 11, 1529.
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kotali, A.; Lafazanis, I.S.; Harris, P.A. 7-Hydroxy-8-acetylcoumarin N-Phenylsulfonylhydrazone. Molbank 2008, 2008, M570. https://doi.org/10.3390/M570
Kotali A, Lafazanis IS, Harris PA. 7-Hydroxy-8-acetylcoumarin N-Phenylsulfonylhydrazone. Molbank. 2008; 2008(2):M570. https://doi.org/10.3390/M570
Chicago/Turabian StyleKotali, Antigoni, Ioannis S. Lafazanis, and Philip A. Harris. 2008. "7-Hydroxy-8-acetylcoumarin N-Phenylsulfonylhydrazone" Molbank 2008, no. 2: M570. https://doi.org/10.3390/M570
APA StyleKotali, A., Lafazanis, I. S., & Harris, P. A. (2008). 7-Hydroxy-8-acetylcoumarin N-Phenylsulfonylhydrazone. Molbank, 2008(2), M570. https://doi.org/10.3390/M570



