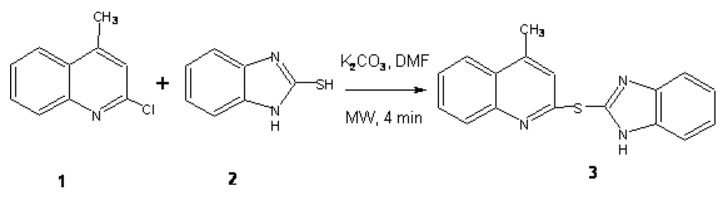
Quinoline derivatives posses wide class of biological activities [1,2,3,4,5]. Microwave heating has emerged as a powerful technique to promote a variety of chemical reaction is due to the short reaction time and the operational simplicity. So number of research paper has appeared proving the synthetic utility of MORE (Microwave-induced Organic Reaction Enhancement) chemistry in routine organic synthesis [6].
2-Chloro-4methylquinoline 1 (708 mg, 0.004 mol) and 2-mercaptobenzimidazole 2 (600 mg, 0.004 mol) were dissolved in minimum amount of anhydrous DMF. To this (552 mg, 0.004 mol) K2CO3 added, then the whole contents was irradiated under microwave oven for about 4 minutes at an interval of 1 min at 160 W. After the completion of reaction (monitored by TLC. ethyl acetate, pet ether 20:80), the reaction mixture was poured into ice-cold water. The obtained greenish yellow colour solid was filtered, washed with water then recrystallised from aqueous DMF, gaves 2-(1H-benzimidazol-2-ylthio)-4-methylquinoline 90 % yield.
Melting Point: 130-132 oC
MS (m/z, %): 292 ([M+H]+, 100%).
1H NMR (400 MHz, DMSO-d6) ¦Ä (ppm): 2.45 (3H, s, Ar-CH3), 7.22 (bs, 1H, NH), 6.90-7.92 (m, 9H, Ar−H), IR (KBr) ¦Í (cm−1): 3150 (N-H), 1250 (C-S-C), 1650 (C=N).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Althuis, T.H.; Khadin, S.B.; Czuba, L.J.; Moore, P.F.; Hess, H.J. J. Med. Chem. 1980, 23, 262. [CrossRef]
- Jiang, J.B.; Isaacson, D. U S Patent 1987, 4656274; Chem. Abstr. 1987, 107, 39643,
- Graeve, R.E.; Pociask, J.R.; Stein, R.G. U S Patent 1971, 3600393.
- Farghaly, A.M.; Habib, N.S.; Khalil, M.A.; El-Sayed, O.A.; Alaxandria, A. J. Pharm. Sci. 1989, 3, 90.Farghaly, A.M.; Habib, N.S.; Khalil, M.A.; El-Sayed, O.A.; Alaxandria, A. Chem. Abstr. 1990, 112, 7420.
- Zikan, V.; Radl, S.; Smejkal, F.; Zelena, D. Czech. Patent 1986, 233445; Chem. Abstr. 1987, 106, 138447,
- Caddick, S. Tetrahedron 1995, 51, 10403. [PubMed]Katritzky, A.R.; Sandeep, K.S. ARKIVOC 2003, 13, 68. [PubMed]
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.