Synthesis and Physical Characterization of 4-(anthracen-10-yl)-1-(4-mthoxyphenyl)-3-phenoxyazetidin-2-one as a New Cis 2-azetidinone
Abstract
:Introduction
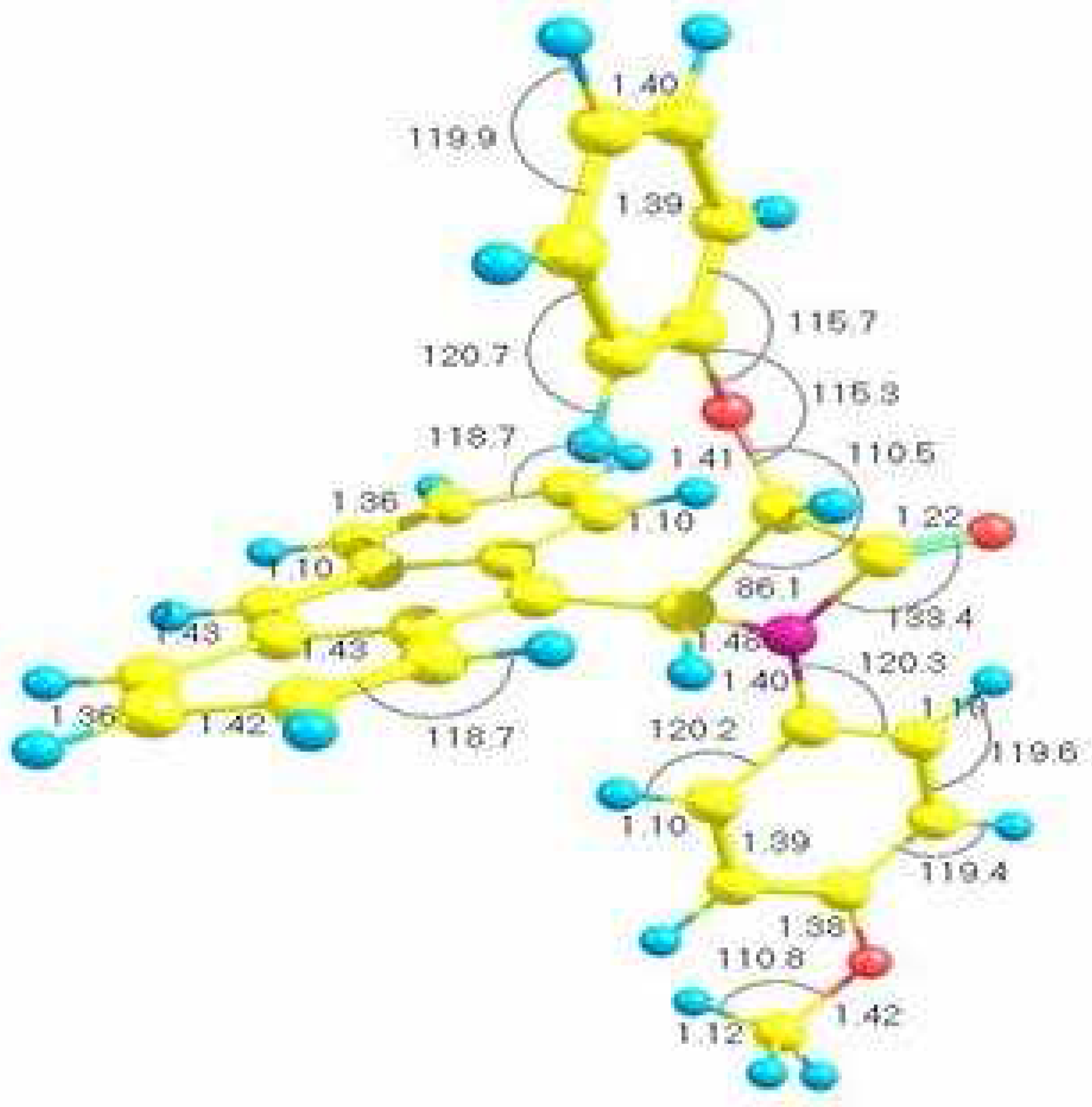
Results and Discussion
Experimental
Synthesis of (E)-N-(antheracen-10-ylmethylene) methoxybenzenamine (1):
Synthesis of 4-(anthracen-10-yl)-1-(4-methoxyphenyl)-3-phenoxy-2- azetidinone (2):
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References
- Yang, J.M.; Cha, S.; Carlson, K.H. J. Chromatogr. A 2006, 1115(1-2), 46–57.
- Jacoby, G.A.; Munoz-Price, L.S. N. Engl. J. Med. 2005, 352, 380–391. [CrossRef] [PubMed]
- Alcaide, B.; Almendros, P.; Pardo, C.; Rodríguez-Vicente, A.; Pilar Ruiz, M. Tetrahedron 2005, 61, 7894–7906.
- Alcaide, B.; Miranda, M.; Perez-Castelles, J.; Sierra, M.A. J. Org. Chem 1993, 58, 297–298.(b)Hess, M. Ring Enlargement in Organic Chemistry; VCH Verlagsgesellschaft: D-6940 Weinheim (Federal Republic of Germany).Manhas, M. S.; Wagle, D. R.; Chiang, J.; Bose, A. K. Heterocycles 1988, 27, 1755–1802.Manhas, M. S.; Amin, S. G.; Bose, A. K. Heterocycles 1976, 5, 669–699.Mukherjee, A.K.; Singh, A.K. Synthesis 1975, 547–589.Deshmukh, A.R.A.S.; Bhawal, B.M.; Krishnaswamy, D.; Govande, V.V.; Shinkre, B.A.; Jayanthi, A. Curr. Med. Chem. 2004, 11, 1889–1920.Alcaide, B.; Almendros, B. Curr. Med. Chem. 2004, 11, 1921–1949. [PubMed]
- Frisch, M. J. GAUSSIAN03. Revision A.1, M. J. Frisch; Gaussian, Inc.: Pittsburgh PA, 2003. [Google Scholar]
- Foresman, J.B. Æ Frisch, Exploring Chemistry with Electronic Structure Methods, 2nd editionGaussian, INC: Pittsburgh, PA, 1996. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. (Eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69. July 2001; National Institute of Standards and Technology: Gaithersburg, MD 20899.
- Jalbout, A.F.; Solimannejad, M.; Labonowski, J.K. Chem. Phys. Letts. 2003, 379, 503. [CrossRef]
- Jalbout, A.F.; Jiang; Jeghnou, H.; Rhandour, A. Vib. Spect. 2006, in press.
- Jalbout, A.F.; Nazara, F.; Turker, L. J. Mol. Struct. (THEOCHEM) 2004, 627, 1, (Invited Review). [CrossRef]

| Cp | -34.41663+ 1842.11254*t -747.52658*t2 +2.83512 *t3 +0.37825 *t-2 | |
| S | -147.10429*ln(t) +2232.95888 *t -1048.98298*t2/2 -15.90654 *t3/3 -1.6256/(2*t2) +0.66663 | |
| ΔH | 13.18293*t +129.55004*t2/2+1145.45566*t3/-151.20546*t4/4 +0.45831/t -0.28471 |
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.
Share and Cite
Jarrahpour, A.; Nazari, M.; Jalbout, A.F. Synthesis and Physical Characterization of 4-(anthracen-10-yl)-1-(4-mthoxyphenyl)-3-phenoxyazetidin-2-one as a New Cis 2-azetidinone. Molbank 2007, 2007, M539. https://doi.org/10.3390/M539
Jarrahpour A, Nazari M, Jalbout AF. Synthesis and Physical Characterization of 4-(anthracen-10-yl)-1-(4-mthoxyphenyl)-3-phenoxyazetidin-2-one as a New Cis 2-azetidinone. Molbank. 2007; 2007(2):M539. https://doi.org/10.3390/M539
Chicago/Turabian StyleJarrahpour, Aliasghar, Mohammad Nazari, and Abraham F. Jalbout. 2007. "Synthesis and Physical Characterization of 4-(anthracen-10-yl)-1-(4-mthoxyphenyl)-3-phenoxyazetidin-2-one as a New Cis 2-azetidinone" Molbank 2007, no. 2: M539. https://doi.org/10.3390/M539
APA StyleJarrahpour, A., Nazari, M., & Jalbout, A. F. (2007). Synthesis and Physical Characterization of 4-(anthracen-10-yl)-1-(4-mthoxyphenyl)-3-phenoxyazetidin-2-one as a New Cis 2-azetidinone. Molbank, 2007(2), M539. https://doi.org/10.3390/M539




