Pyridazines are of chemical and biological interest. They have been reported to be anticonvulsive agents [1], [2]. Furthermore, BELLASIO et al. have described the antihypertensive effects of hydrazinopyridazine compounds [3]. In continuation of this line of investigation, we have synthesized compound (I); it will be subjected to further pharmacological investigations, especially tests of its anticancer activity.
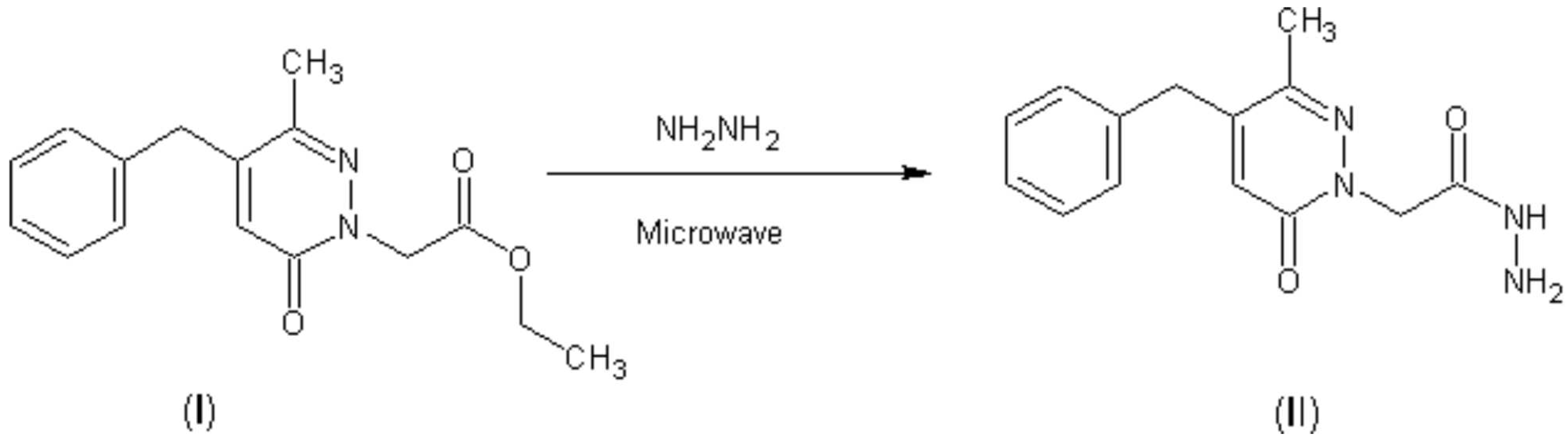
To (0.86 g, 3 mmol) of ethyl (4-benzyl-3-methyl-6-oxopyridazin-1(6H)-yl)acetate (I), was added 10 ml of hydrazine hydrate. The mixture was placed in a pyrex tube which was then introduced into a Maxidigest MX 350 Prolabo microwave [4] monomode reactor and refluxed for 10 min on 60 w as irradiation power. After cooling, the product precipitates, and then is recrystallised in absolute ethanol, yield: 85 % of (II) solid.
Melting point: 197-200°C
IR (KBr, cm-1): 3350 (NH), 1680, 1620 (C = O)
1HNMR (300.14 MHz, CDCl3) δ (ppm): 2.50 (s, 3H, CH3). 2.53 (s, 2H, NH2), 3.79 (s, 2H, CH2), 4.84 (d, 2H, CH2), 6.54 (s, 1H, H4), 7.31 (m, 5H, H aromatic), 7.73 (s, 2H, NH2).
13CNMR (75 MHz, CDCl3) δ (ppm): 19.55 (CH3), 38.82 (CH2), 54.73 (CH2), 127.77 (CH aromatic), 128.26 (CH aromatic), 129.48 (2 CH aromatic), 135.77, 146.50, 147.34, 161.09 (C=O), 168.32 (C=O).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Wermuth, C.G.; Leclerc, G.; Melounov, P. Chim. Ther. 1971, 2, 109.
- Laborit, H.; Weber, B.; Wermuth, C.G.; Delbarre, B.; Chekler, C.; Baron, C.; Rosen Garten, H. AGRESSOLOGIE 1965, 6, 415. [PubMed]
- Bellasio, E.; Parravicini, F.; Testa, E. Il Farmaco Ed Sci. 1969, 11, 919.
- (a) Kappe, C.O.; Dallinger, D. Nature Reviews Drug Discovery 2006, 5, 51. (b) De la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Chem. Soc. Rev. 2005, 34, 164.
© 2007 By MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.