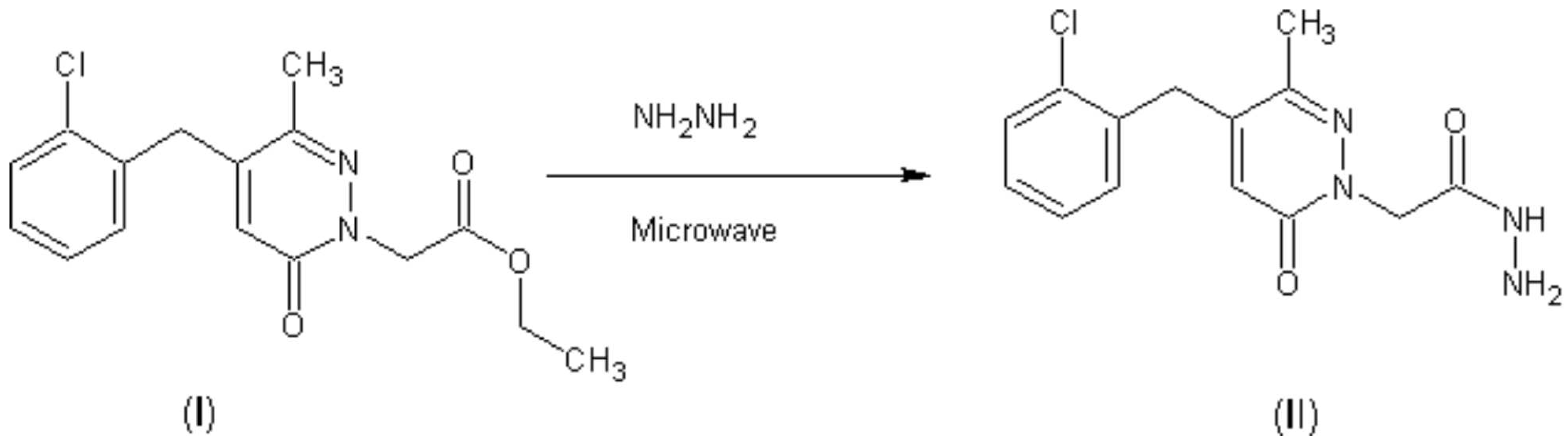
Synthesis of 2-[4-(2-chlorobenzyl)-3-methyl-6-oxopyridazin-1(6H)-yl]acetohydrazide
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Wermuth, C.G.; Leclerc, G.; Melounov, P. Chim. Ther. 1971, 2, 109.
- Laborit, H.; Weber, B.; Wermuth, C.G.; Delbarre, B.; Chekler, C.; Baron, C.; Rosen Garten, H. AGRESSOLOGIE 1965, 6, 415. [PubMed]
- Bellasio, E.; Parravicini, F.; Testa, E. Il Farmaco Ed Sci. 1969, 11, 919.
- (a) Kappe, C.O.; Dallinger, D. Nature Reviews Drug Discovery 2006, 5, 51. (b) De la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Chem. Soc. Rev. 2005, 34, 164.
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.
Share and Cite
Benchat, N.-E.; Anaflous, A.; Abouricha, S.; El-Bali, B.; Ramdani, M. Synthesis of 2-[4-(2-chlorobenzyl)-3-methyl-6-oxopyridazin-1(6H)-yl]acetohydrazide. Molbank 2007, 2007, M531. https://doi.org/10.3390/M531
Benchat N-E, Anaflous A, Abouricha S, El-Bali B, Ramdani M. Synthesis of 2-[4-(2-chlorobenzyl)-3-methyl-6-oxopyridazin-1(6H)-yl]acetohydrazide. Molbank. 2007; 2007(2):M531. https://doi.org/10.3390/M531
Chicago/Turabian StyleBenchat, Nour-Eddine, Abderrahmane Anaflous, Said Abouricha, Brahim El-Bali, and Mohamed Ramdani. 2007. "Synthesis of 2-[4-(2-chlorobenzyl)-3-methyl-6-oxopyridazin-1(6H)-yl]acetohydrazide" Molbank 2007, no. 2: M531. https://doi.org/10.3390/M531
APA StyleBenchat, N.-E., Anaflous, A., Abouricha, S., El-Bali, B., & Ramdani, M. (2007). Synthesis of 2-[4-(2-chlorobenzyl)-3-methyl-6-oxopyridazin-1(6H)-yl]acetohydrazide. Molbank, 2007(2), M531. https://doi.org/10.3390/M531



