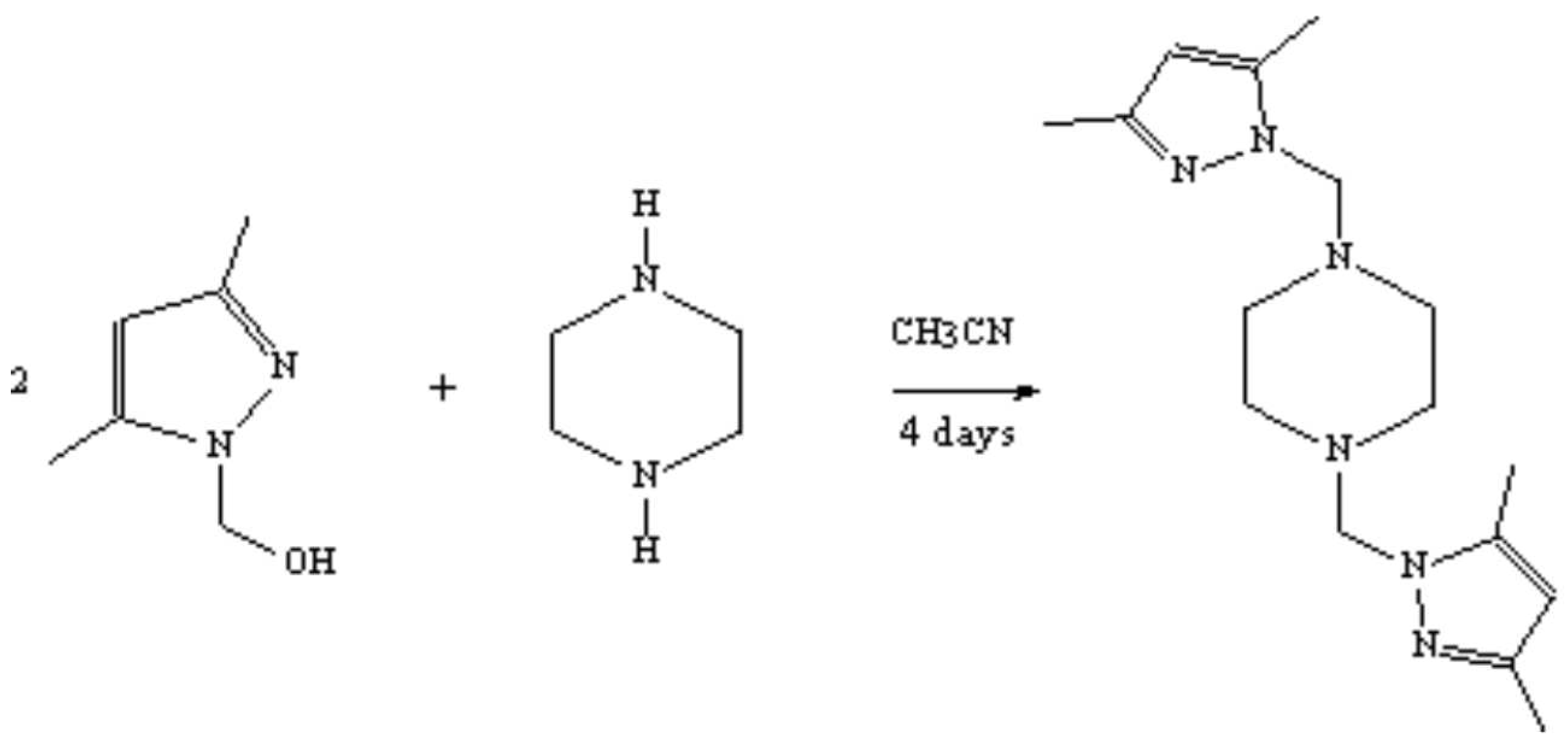
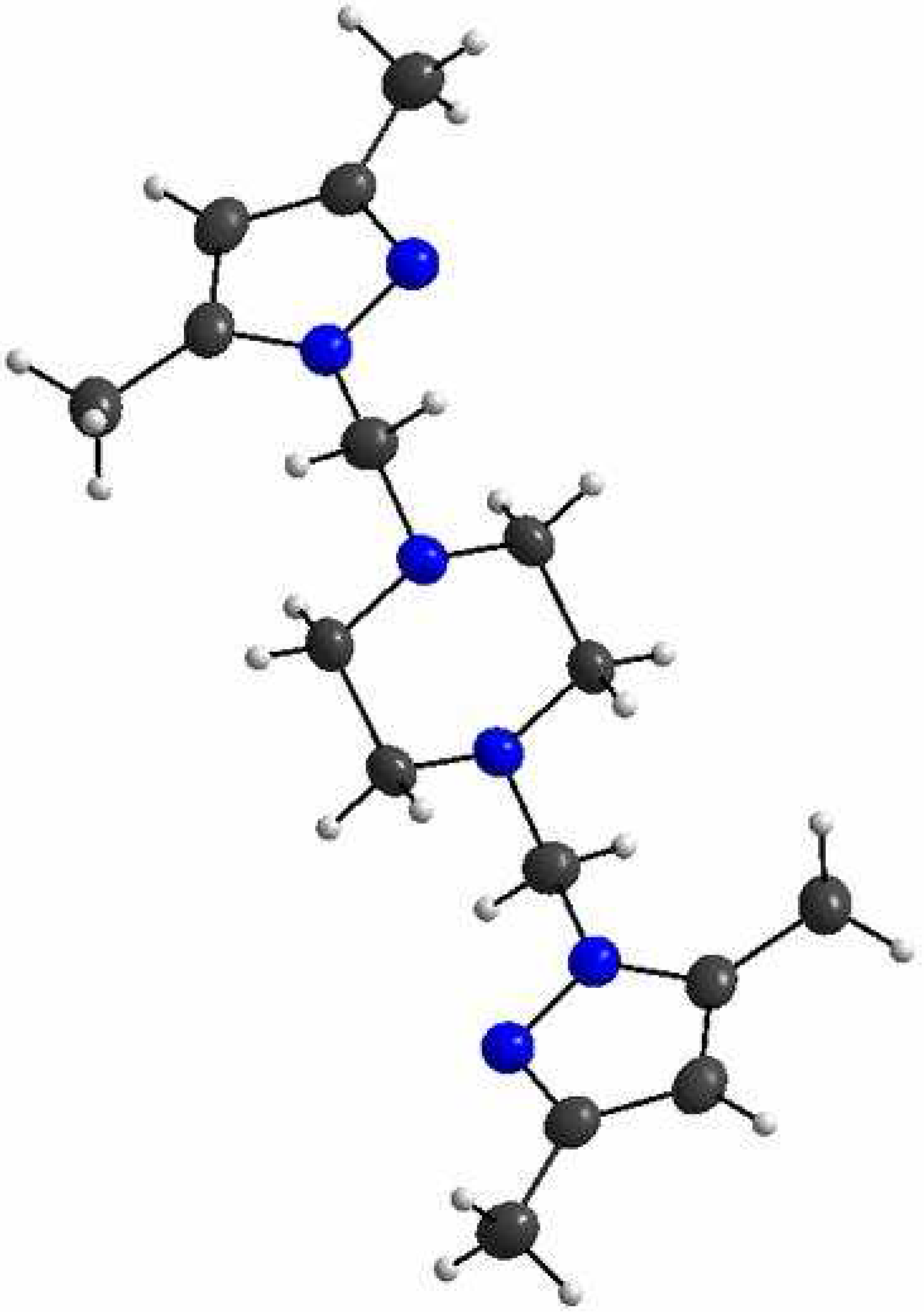
The product 2 was prepared by the addition of piperazine (C4H10N2) to 1 [1] according to the reported procedure [2]. To a solution of the substituted hydroxymethylpyrazole 1 (1.26 g, 10 mmol) in acetonitrile (50 ml) was added piperazine (0.95 g, 5 mmol) and the mixture was stirred. The stirring was continued at room temperature for 4 days. The solvent was evaporated under reduced pressure. The solid was cristallised in ethanol to afford 2 as a white solid (2.31 g, 76%).
Melting point: 158-160°C.
IR (KBr, cm– 1): 2270 (CH); 1650 (C=C, C=N).
1H- NMR (60 MHz, CDCl3): δ= 5 .8 (s, 3H, Pyrazol, H4,4'); 4.6 (s, 4H, 2NCH2N); 2.6 (s, 8H, 4CH2-N); 2.30 (s, 6H, 2CH3).
13C-NMR (300MHz, D2O): δ= 9.64 (Pz-CH3); 12.12 (Pz-CH3); 69.4 (Pz-CH2-N); 81.75 (N-CH2-CH2 N); 106.38(PzC-H); 141.66 (PzC=N); 149.94(PzC-N).
EI-MS (m/z; %): Calculated for C16H26N6 : 302.418. Found: 303[M+]; 109; 95.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
We are indebted to the "Projet Global d’Université Mohamed Premier" for financial support.
References
- Dvoretzky, I.; Richter, G.H. J.Org.Chem. 1950, 15, 1285.
- Sheu, S-C.; Tien, M-J.; Cheng, M-C.; Ho, T-I.; Peng, S-M.; And Lin, Y-C. J. Chem. Soc. Dalton Trans 1995, 3503–3510.
- Sample Availability: Available from the authors and MDPI.
© 2006 MDPI. All rights reserved.