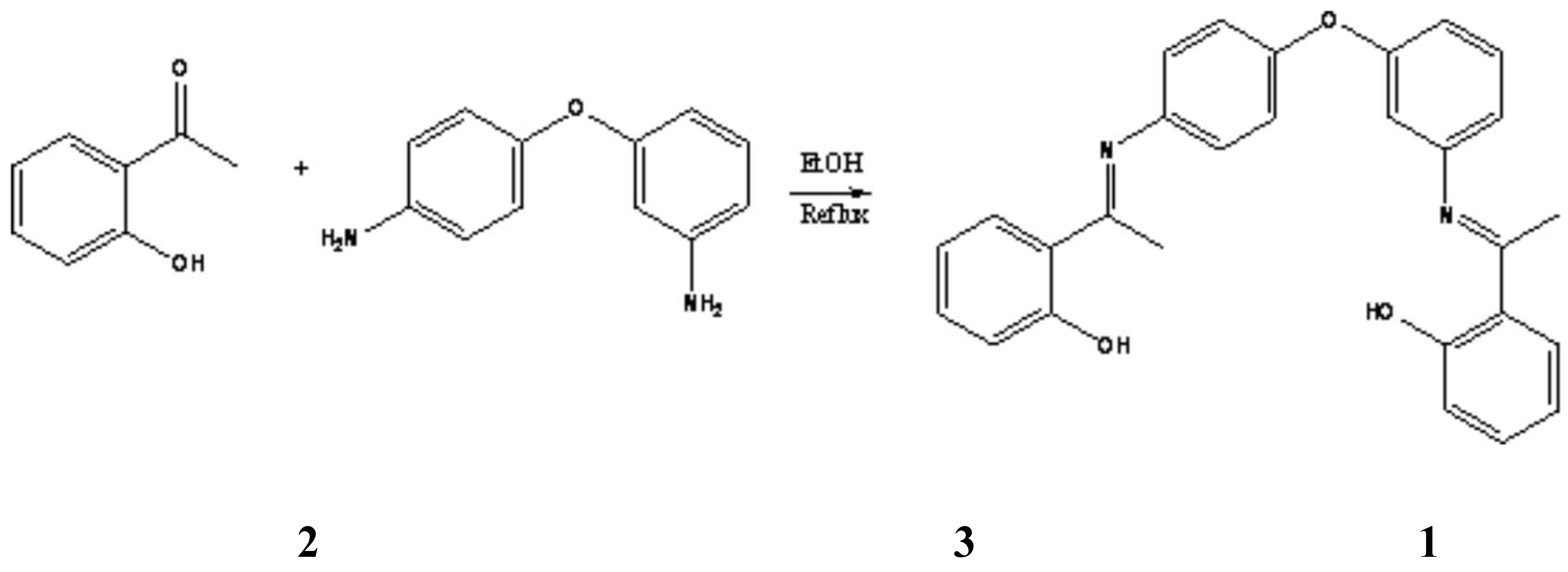
Synthesis of N,N'-bis (a-methylsalicylidene)-3,4'-diaminodiphenyl ether
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References
- Zhang, J. X.; Zhou, Y.; Cai, G. J. Mol. Catal. 1997, 11, 41–44.Holland, D.; Laidler, D.A.; Milner, D.J. J. Mol. Catal. 1981, 11, 119–127.Chen, G. M.; Chen, F.; Zhou, C. Chem. Chin. Univ 1995, 16, 216–219.Qiu, M.; Liu, G.; Yiao, X. Chin. J. Catal. 2001, 22, 77–80.Li, C.; Zhang, W.; Yao, X. Chin. J. Catal. 2000, 21, 77–80.Li, Z. N.; Liu, G.; Zheng, Z. Tetrahedron 2000, 56, 7187–7191.
- Gao, W. T.; Zheng, Z. Molecules 2002, 7, 511–516.
- Tajkhorshid, E.; Paizs, B.; Suhai, S. J. Phys. Chem 1997, 101, 8021–8027.
- Ruck, R. T.; Jacobsen, E. J. J. Am. Chem. Soc. 2002, 124, 2882–2883.
- Sample Availability: Available from MDPI.
© 2006 MDPI. All rights reserved.
Share and Cite
Jarrahpour, A.A.; Rezaei, S. Synthesis of N,N'-bis (a-methylsalicylidene)-3,4'-diaminodiphenyl ether. Molbank 2006, 2006, M458. https://doi.org/10.3390/M458
Jarrahpour AA, Rezaei S. Synthesis of N,N'-bis (a-methylsalicylidene)-3,4'-diaminodiphenyl ether. Molbank. 2006; 2006(1):M458. https://doi.org/10.3390/M458
Chicago/Turabian StyleJarrahpour, A. A., and S. Rezaei. 2006. "Synthesis of N,N'-bis (a-methylsalicylidene)-3,4'-diaminodiphenyl ether" Molbank 2006, no. 1: M458. https://doi.org/10.3390/M458
APA StyleJarrahpour, A. A., & Rezaei, S. (2006). Synthesis of N,N'-bis (a-methylsalicylidene)-3,4'-diaminodiphenyl ether. Molbank, 2006(1), M458. https://doi.org/10.3390/M458



