Synthesis of 1-(2-aminopyridine)-4-phenyl-1-azabuta-1,3-diene and 1-(3-aminopyridine)-4-phenyl-1-azabuta-1,3-diene as heterodienes for iron carbonyl complexes
Abstract
:Introduction
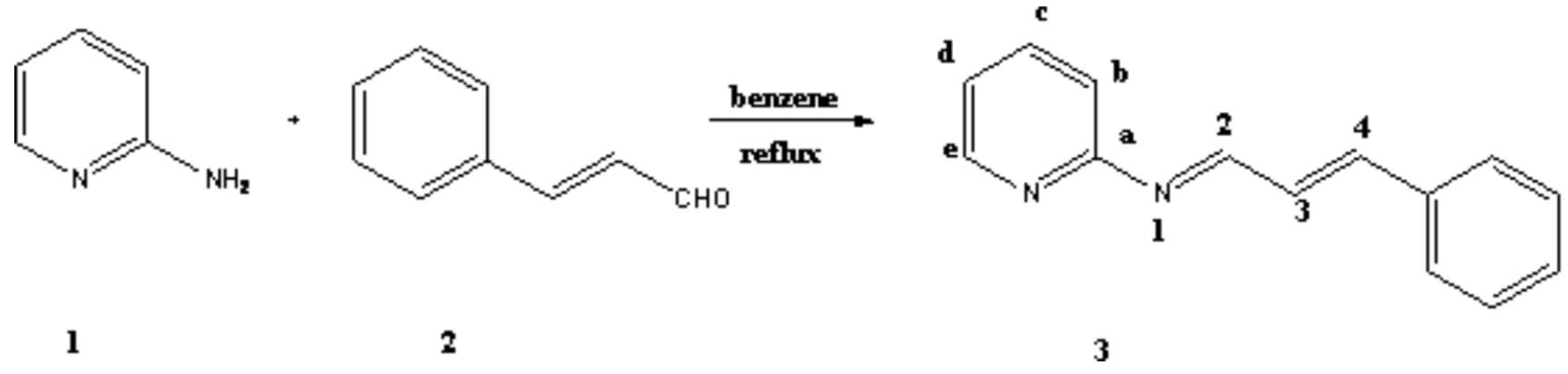
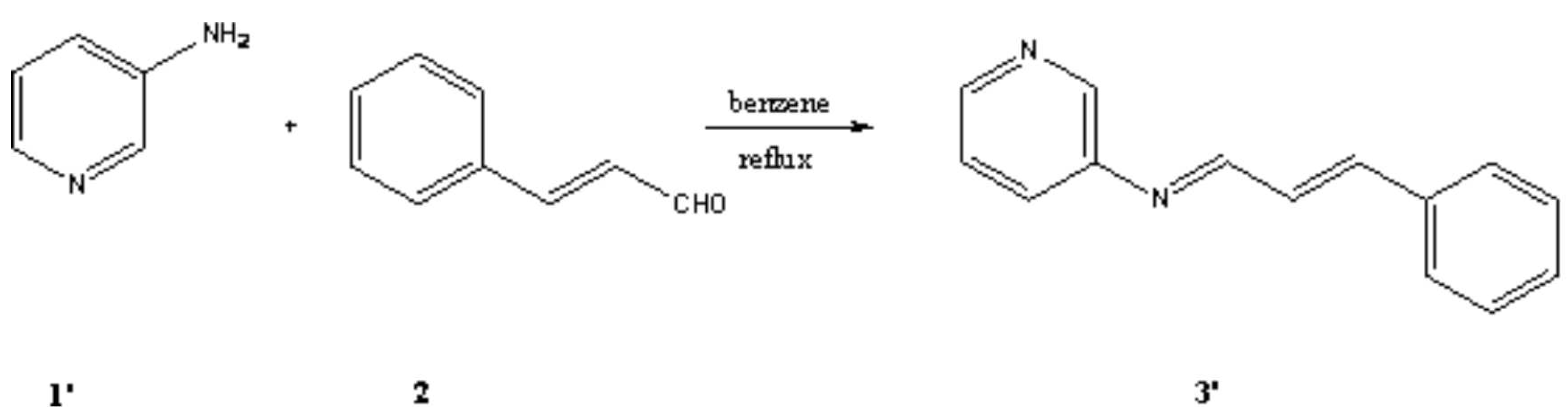
Results and Discussion
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References
- Coates, G. E.; Green, M. L. H.; Wade, K. Organometallic Compounds; Methuen, London, 1969; Vol II, p. 65. [Google Scholar]
- Dieck, H. T.; Bock, H. Chem. Comm. 1968, 678.
- Maywald, F.; Eilbracht, P. Synlett. 1996, 380–382.
- Wrackmeyer, B.; Seidel, G.; Köster, R. Magnetic Resonance in Chemistry 2000, 38 (7), 520–524. [PubMed]
- Kuramshin, A. I.; Kuramshina, E. A.; Cherkasov, R. A. Russ. J. Org. Chem. 2005, 41 (5), 649–655.
- Sample Availability: Available from MDPI.
© 2006 MDPI. All rights reserved.
Share and Cite
Jarrahpour, A.A.; Esmaeilbeig, A.R.; Adabi Ardekani, A. Synthesis of 1-(2-aminopyridine)-4-phenyl-1-azabuta-1,3-diene and 1-(3-aminopyridine)-4-phenyl-1-azabuta-1,3-diene as heterodienes for iron carbonyl complexes. Molbank 2006, 2006, M457. https://doi.org/10.3390/M457
Jarrahpour AA, Esmaeilbeig AR, Adabi Ardekani A. Synthesis of 1-(2-aminopyridine)-4-phenyl-1-azabuta-1,3-diene and 1-(3-aminopyridine)-4-phenyl-1-azabuta-1,3-diene as heterodienes for iron carbonyl complexes. Molbank. 2006; 2006(1):M457. https://doi.org/10.3390/M457
Chicago/Turabian StyleJarrahpour, A. A., A. R. Esmaeilbeig, and A. Adabi Ardekani. 2006. "Synthesis of 1-(2-aminopyridine)-4-phenyl-1-azabuta-1,3-diene and 1-(3-aminopyridine)-4-phenyl-1-azabuta-1,3-diene as heterodienes for iron carbonyl complexes" Molbank 2006, no. 1: M457. https://doi.org/10.3390/M457
APA StyleJarrahpour, A. A., Esmaeilbeig, A. R., & Adabi Ardekani, A. (2006). Synthesis of 1-(2-aminopyridine)-4-phenyl-1-azabuta-1,3-diene and 1-(3-aminopyridine)-4-phenyl-1-azabuta-1,3-diene as heterodienes for iron carbonyl complexes. Molbank, 2006(1), M457. https://doi.org/10.3390/M457



