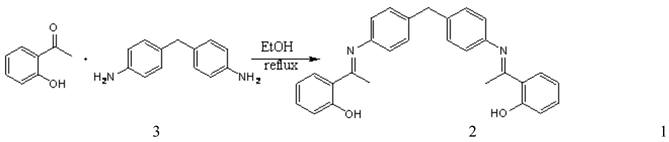
Synthesis of N,N'-bis (a-methylsalicylidene) 4,4'-diaminodiphenylmethane as a novel complexing agent
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References:
- Gama, A.; Flores-López, L. Z.; Aguirre, G.; Hellberg, M. P. H.; Somanathan, R. Arkivoc 2003, 4–15.
- Cameron, P. A.; Gibson, V. C.; Redshaw, C.; Segal, J. A.; Bruce, M. D.; White, A.; Williams, D. Chem. Commun. 1999, 1883–1884.
- Szłyk, E.; Barwiołek, M.; Kruszynski, R.; Bartczak, T. Inorg. Chim. Acta 2005, 358, 3642–3652.
- Flores-lopez, L. Z.; Parra-Hake, M. P.; Somanathan, R.; Walsh, P. J. Organometallics 2000, 19, 2153–2160.
- Kervinen, K.; Korpi, H.; Leskela, M.; Repo, T. J. Mol. Cat. 2003, 203, 9–19.
- Sample Availability: Available from MDPI.
© 2006 MDPI. All rights reserved.
Share and Cite
Jarrahpour, A.A.; Rezaei, S. Synthesis of N,N'-bis (a-methylsalicylidene) 4,4'-diaminodiphenylmethane as a novel complexing agent. Molbank 2006, 2006, M456. https://doi.org/10.3390/M456
Jarrahpour AA, Rezaei S. Synthesis of N,N'-bis (a-methylsalicylidene) 4,4'-diaminodiphenylmethane as a novel complexing agent. Molbank. 2006; 2006(1):M456. https://doi.org/10.3390/M456
Chicago/Turabian StyleJarrahpour, A. A., and S. Rezaei. 2006. "Synthesis of N,N'-bis (a-methylsalicylidene) 4,4'-diaminodiphenylmethane as a novel complexing agent" Molbank 2006, no. 1: M456. https://doi.org/10.3390/M456
APA StyleJarrahpour, A. A., & Rezaei, S. (2006). Synthesis of N,N'-bis (a-methylsalicylidene) 4,4'-diaminodiphenylmethane as a novel complexing agent. Molbank, 2006(1), M456. https://doi.org/10.3390/M456



