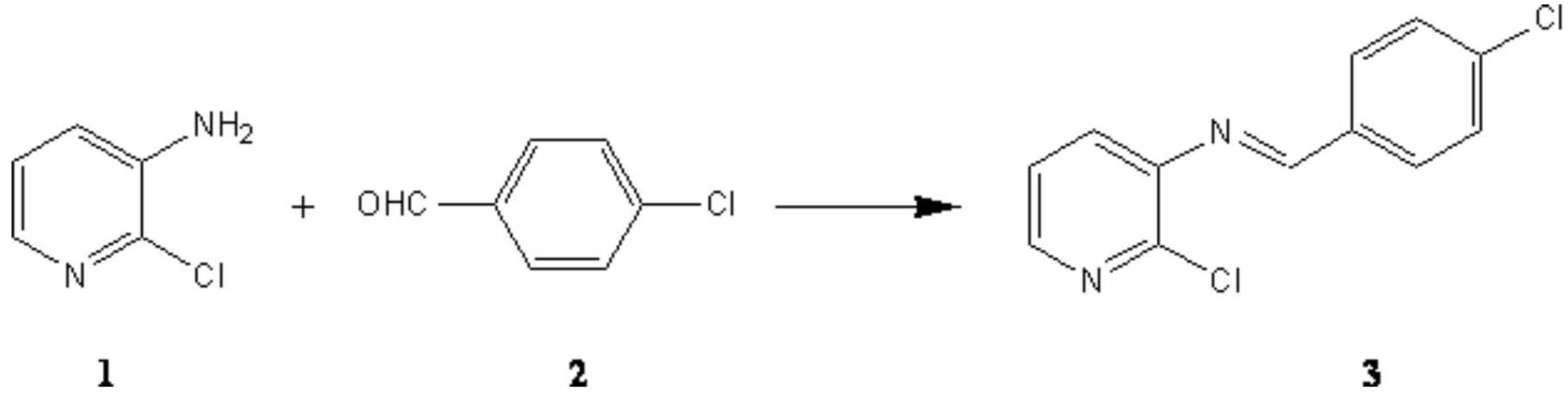
Synthesis of (4-chlorobenzylidene)-(2-chloropyridi-3-yl)amine
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References
- Arora, K.; Gupta, A.; Agarwall, D. D. Asian. J. Chem. 2002, 14, 1611.
- Tanaka, K.; Shiraishi, R. Green Chem. 2000, 2, 272.
- Shaikh kabeer, A.; Baseer, M. A.; Mote, N. A. Asian J. Chem. 2001, 13(2), 496.
- Pandeya, S. N.; Sriram, D.; Nath, G.; De Clercq, E. Il Farmaco 1999, 54, 624. [PubMed]
- El-masry, Afaf. H.; Fahmy, H. H.; Ali Abdelwahed, S. H. Molecules 2000, 5, 1429.
- More, P. G.; Bhalvankar, R. B.; Pattar, S. C. J. Indian Chem. Soc. 2001, 78, 474.
- Singh, W. M.; Dash, B. C. Pesticides 1988, 22(11), 33.
- Desai, S. B.; Desai, P. B.; Desai, K. R. Hetrocycl. Commun. 2001, 7(1), 83.
- Phatak, P.; Jolly, V. S.; Sharma, K. P. Orient. J. Chem. 2000, 16(3), 493.
- Samadhiya, S.; Halve, A. Orient. J. Chem. 2001, 17(1), 119.
- Sepcic, Kristina. J. Toxicol., Toxin Rev. 2000, 19(2), 139.
- Sliwa, W.; Mianowska, B. Hetrocycles 1989, 29(3), 557.
- Durta, I.; Dinica, R.; Bacu, E.; Anderi, M. Chem. Abstr. 2000, 132, 205326b.
- Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.
Share and Cite
Jarrahpour, A.A.; Zarei, M. Synthesis of (4-chlorobenzylidene)-(2-chloropyridi-3-yl)amine. Molbank 2005, 2005, M438. https://doi.org/10.3390/M438
Jarrahpour AA, Zarei M. Synthesis of (4-chlorobenzylidene)-(2-chloropyridi-3-yl)amine. Molbank. 2005; 2005(4):M438. https://doi.org/10.3390/M438
Chicago/Turabian StyleJarrahpour, A. A., and M. Zarei. 2005. "Synthesis of (4-chlorobenzylidene)-(2-chloropyridi-3-yl)amine" Molbank 2005, no. 4: M438. https://doi.org/10.3390/M438
APA StyleJarrahpour, A. A., & Zarei, M. (2005). Synthesis of (4-chlorobenzylidene)-(2-chloropyridi-3-yl)amine. Molbank, 2005(4), M438. https://doi.org/10.3390/M438



