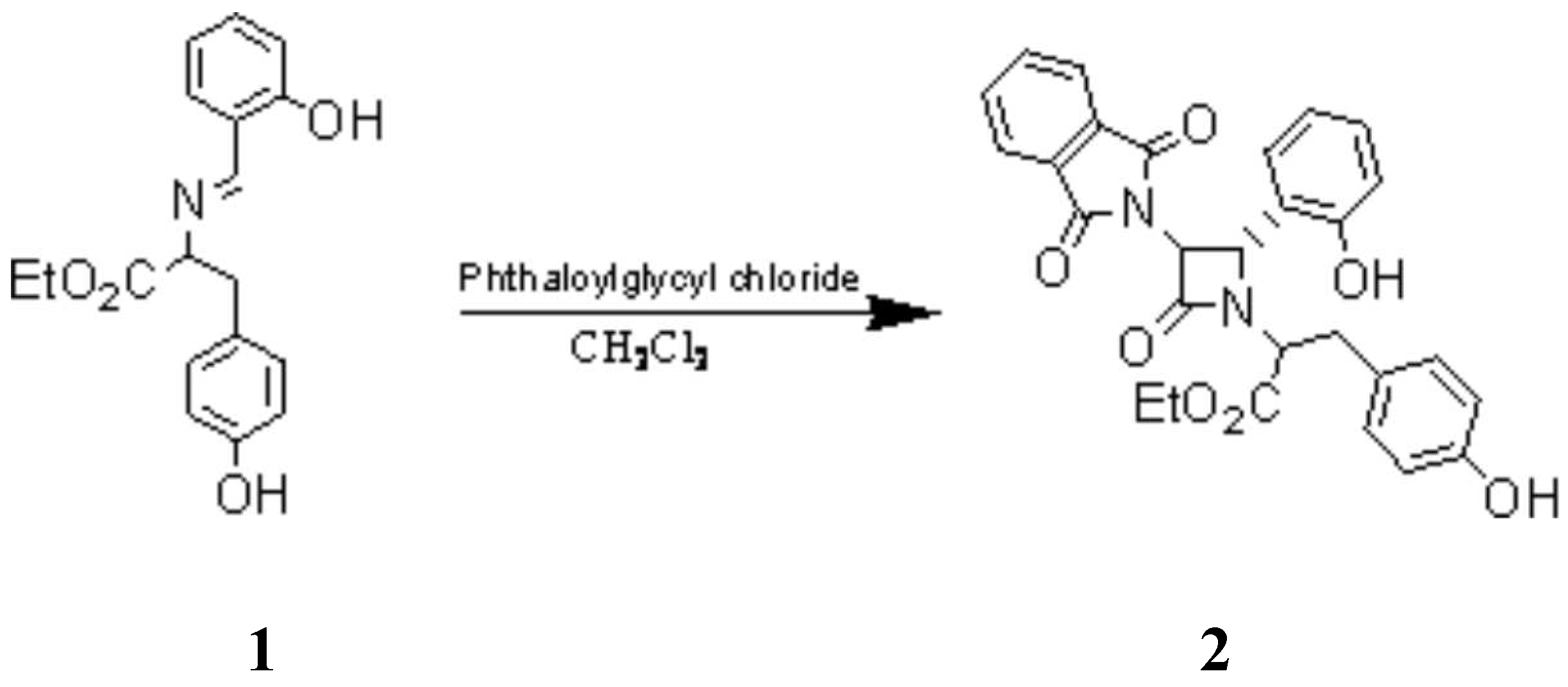
Asymmetric Synthesis of a New Monocyclic β-Lactam as a potential biological active compound
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References
- Jarrahpour, A. A.; Shekarriz, M.; Taslimi, A. Molecules 2004, 9, 29.Jarrahpour, A. A.; Shekarriz, M.; Taslimi, A. Molecules 2004, 9, 939.
- Palomo, C.; Aizpurua, J. M.; Ganboa, I.; Oiarbide, M. Eur. J. Org. Chem. 1999, 3223.
- Palomo, C.; Aizpurua, J. M.; Ganboa, I.; Oiarbide, M. Amino Acids 1999, 16, 321. [PubMed]
- Ojima, I.; Chen, H. J. C.; Qiu, X. Tetrahedron 1988, 44(17), 5307.
- Van der Steen, F. H.; Van Koten, G. Tetrahedron 1991, 47, 7503.
- Georg, G. I.; Ravikumar, V. T. The Organic Chemistry of β-Lactams; Georg, G. I., Ed.; WCH: New York, 1993; p. 295. [Google Scholar]
- Hashimoto, Y.; Ogasawara, T.; Hayashi, M.; Saigo, K. Heterocycles 2000, 52, 1001.
- Arunkumar, N.; Keyan, W.; Ramamurthy, V.; Scheffer, J. R.; Brain, P. Org. Lett. 2002, 4, 1443.
- France, S.; Wack, H.; Hafez, A. M.; Taggi, A. E.; Witsil, D. R.; Lectka, T. Org. Lett. 2002, 4, 1603. [PubMed]
- Sample Availability Available from MDPI.
© 2005 MDPI. All rights reserved.
Share and Cite
Jarrahpour, A.A.; Jahaniani, A.R. Asymmetric Synthesis of a New Monocyclic β-Lactam as a potential biological active compound. Molbank 2005, 2005, M439. https://doi.org/10.3390/M439
Jarrahpour AA, Jahaniani AR. Asymmetric Synthesis of a New Monocyclic β-Lactam as a potential biological active compound. Molbank. 2005; 2005(4):M439. https://doi.org/10.3390/M439
Chicago/Turabian StyleJarrahpour, A. A., and A. R. Jahaniani. 2005. "Asymmetric Synthesis of a New Monocyclic β-Lactam as a potential biological active compound" Molbank 2005, no. 4: M439. https://doi.org/10.3390/M439
APA StyleJarrahpour, A. A., & Jahaniani, A. R. (2005). Asymmetric Synthesis of a New Monocyclic β-Lactam as a potential biological active compound. Molbank, 2005(4), M439. https://doi.org/10.3390/M439



