3-Bromomethyl-1,5-diphenyl-1H-pyrazolo[4,3-e][1,2,4]triazine and 3-Dibromomethyl-1,5-diphenyl-1H-pyrazolo[4,3-e][1,2,4]triazine
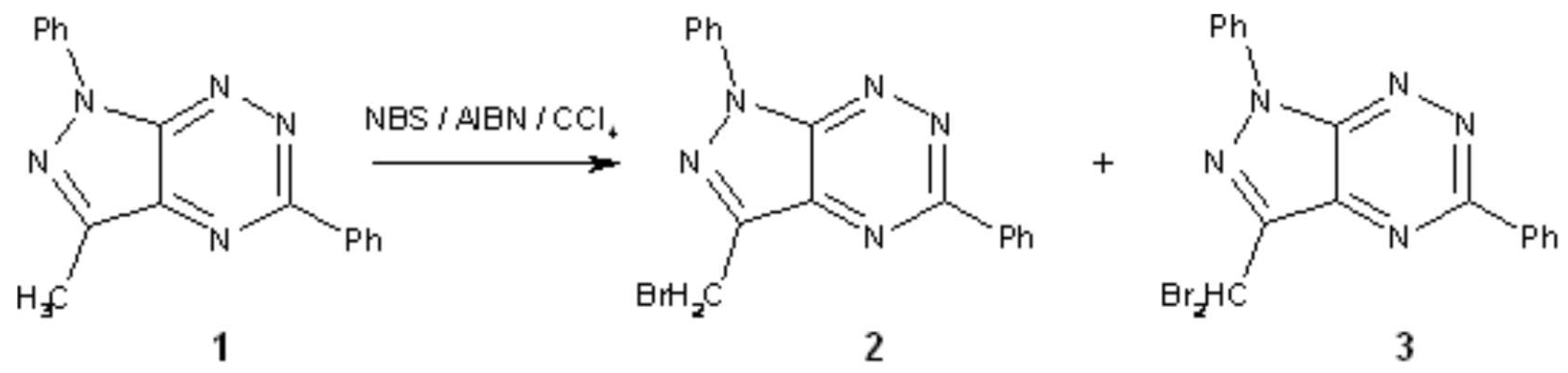
3-Bromomethyl-1,5-Diphenyl-1H-Pyrazolo[4,3-e][1,2,4]Triazine (2)
3-Dibromomethyl-1,5-Diphenyl-1H-Pyrazolo[4,3-e][1,2,4]Triazine (3)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6References
- Rykowski, A.; Mojzych, M.; Karczmarzyk, Z. Heterocycles 2000, 53, 2175.
- Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.
Share and Cite
Mojzych, M. 3-Bromomethyl-1,5-diphenyl-1H-pyrazolo[4,3-e][1,2,4]triazine and 3-Dibromomethyl-1,5-diphenyl-1H-pyrazolo[4,3-e][1,2,4]triazine. Molbank 2005, 2005, M432. https://doi.org/10.3390/M432
Mojzych M. 3-Bromomethyl-1,5-diphenyl-1H-pyrazolo[4,3-e][1,2,4]triazine and 3-Dibromomethyl-1,5-diphenyl-1H-pyrazolo[4,3-e][1,2,4]triazine. Molbank. 2005; 2005(4):M432. https://doi.org/10.3390/M432
Chicago/Turabian StyleMojzych, Mariusz. 2005. "3-Bromomethyl-1,5-diphenyl-1H-pyrazolo[4,3-e][1,2,4]triazine and 3-Dibromomethyl-1,5-diphenyl-1H-pyrazolo[4,3-e][1,2,4]triazine" Molbank 2005, no. 4: M432. https://doi.org/10.3390/M432
APA StyleMojzych, M. (2005). 3-Bromomethyl-1,5-diphenyl-1H-pyrazolo[4,3-e][1,2,4]triazine and 3-Dibromomethyl-1,5-diphenyl-1H-pyrazolo[4,3-e][1,2,4]triazine. Molbank, 2005(4), M432. https://doi.org/10.3390/M432



