N-Furan-2-ylmethyl-4-methyl-benzenesulfonamide (N-Furfuryl-p-toluenesulfonamide)
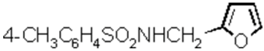
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Commercially available; we used a product from Fluka, Switzerland.
- tert-Butylmethyl ether was recommended as an inexpensive solvent when this experiment was carried out (June 1991, Diploma thesis of H.M.). Nowadays, it is considered environmentally problematic. We also used 1,2-dichloroethane instead of the ether.
- Chooney, N.; Kuhnert, N.; Sammes, P. G.; Smith, G.; Ward, R. W. J. Chem. Soc., Perkin Trans 1 2002, 1999–2005, Dry pyridine was used as base and solvent. However, spectra and combustion analysis are not given in this paper..
© 2004 MDPI. All rights reserved.
Share and Cite
Meining, H.; Föhlisch, B. N-Furan-2-ylmethyl-4-methyl-benzenesulfonamide (N-Furfuryl-p-toluenesulfonamide). Molbank 2004, 2004, M391. https://doi.org/10.3390/M391
Meining H, Föhlisch B. N-Furan-2-ylmethyl-4-methyl-benzenesulfonamide (N-Furfuryl-p-toluenesulfonamide). Molbank. 2004; 2004(1):M391. https://doi.org/10.3390/M391
Chicago/Turabian StyleMeining, Holger, and Baldur Föhlisch. 2004. "N-Furan-2-ylmethyl-4-methyl-benzenesulfonamide (N-Furfuryl-p-toluenesulfonamide)" Molbank 2004, no. 1: M391. https://doi.org/10.3390/M391
APA StyleMeining, H., & Föhlisch, B. (2004). N-Furan-2-ylmethyl-4-methyl-benzenesulfonamide (N-Furfuryl-p-toluenesulfonamide). Molbank, 2004(1), M391. https://doi.org/10.3390/M391



