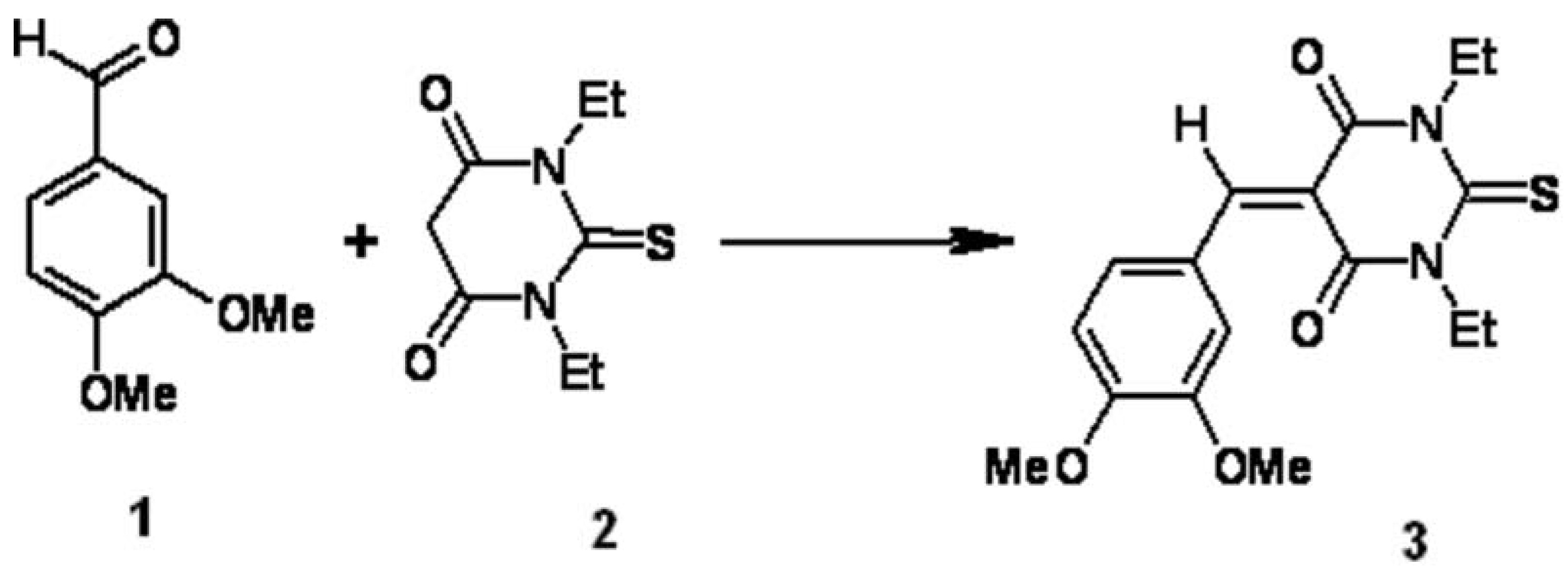
5-(3,4-Dimethoxybenzylidene)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Jones, G. Org. React. 1967, 15, 204.
- Tanaka, K.; Chen, X.; Yoneda, F. Tetrahedron 1988, 44, 3241.
© 2004 MDPI. All rights reserved.
Share and Cite
Asiri, A.M.; Alamry, K.A. 5-(3,4-Dimethoxybenzylidene)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione. Molbank 2004, 2004, M361. https://doi.org/10.3390/M361
Asiri AM, Alamry KA. 5-(3,4-Dimethoxybenzylidene)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione. Molbank. 2004; 2004(1):M361. https://doi.org/10.3390/M361
Chicago/Turabian StyleAsiri, Abdullah Mohamed, and Khaled Ahmed Alamry. 2004. "5-(3,4-Dimethoxybenzylidene)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione" Molbank 2004, no. 1: M361. https://doi.org/10.3390/M361
APA StyleAsiri, A. M., & Alamry, K. A. (2004). 5-(3,4-Dimethoxybenzylidene)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione. Molbank, 2004(1), M361. https://doi.org/10.3390/M361



