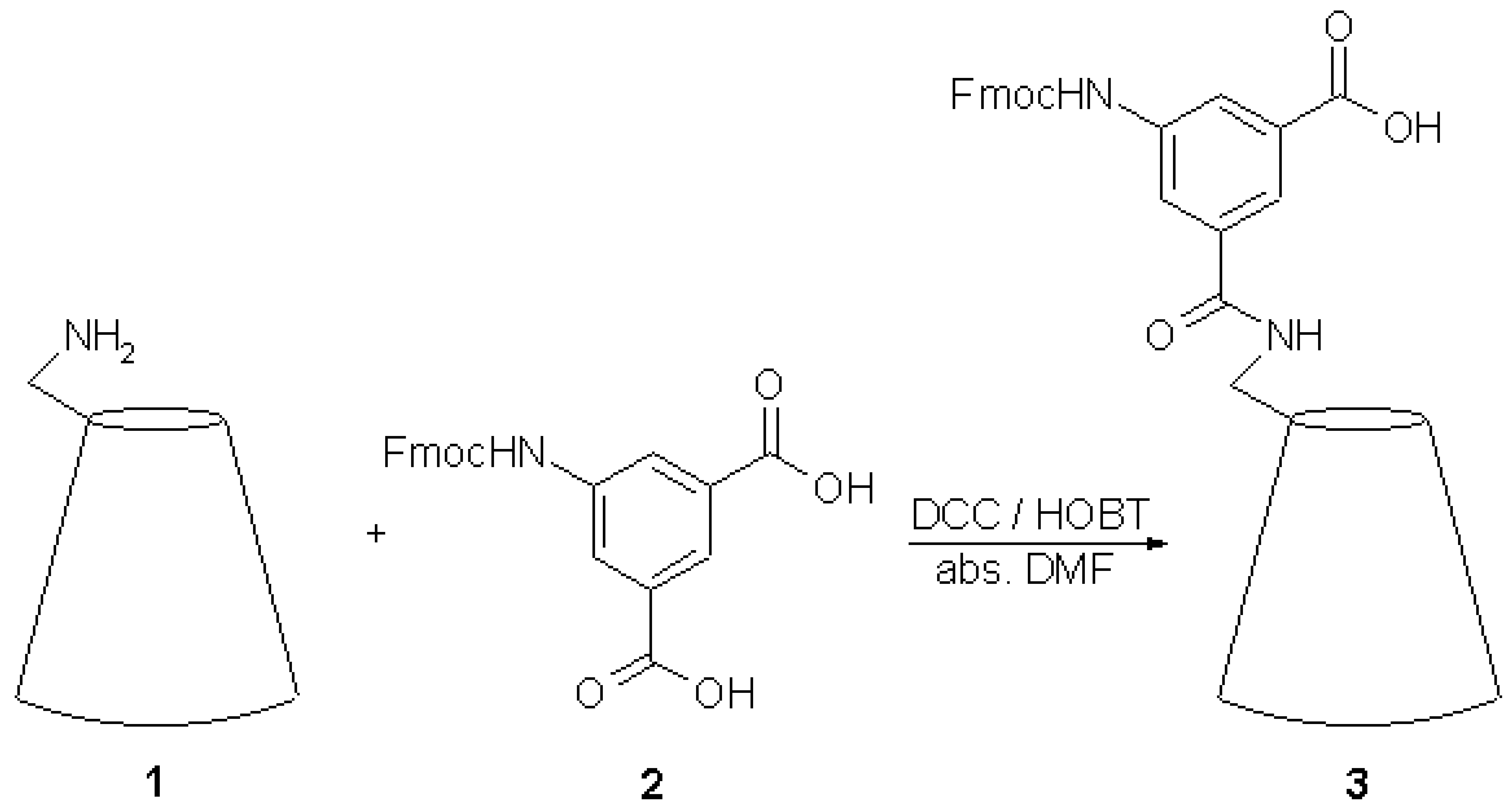
6-Amino-6-deoxy-b-cyclodextrine (
1) was prepared according to the literature [
1,
2] in a three step synthesis. The Fmoc protected 5-aminoisophthalic acid is prepared according to the following procedure. To a solution of 15 ml of dioxane and 40 ml of water containing 5-aminoisophthalic acid (500 mg, 2.76 mmol) and Na
2CO
3 (878 mg, 8.28mmol) a solution of FmocCl (785 mg, 3.04 mmol) in 10 ml of dioxane was added dropwise at 0° within 15 min. The mixture was stirred for 20 h at room temp., 2 M HCl solution was added until a pH of 2 is reached. The crude product was extracted with 250 ml of ethyl acetate. The organic phase was dried over Na
2SO
4 and evaporated under reduced pressure. The residual was then stirred in 100 ml of methylene chloride for 2 h, filtered, washed with methylene chloride and dried under reduced pressure to yield 1.01 g (91%) of crude
2 as a light brown powder.
To obtain the titled product 3 a solution of DCC (66 mg, 0.32 mmol) and HOBT (43 mg, 0.32 mmol) in 20 ml of abs. DMF was added dropwise to a solution of 2 (118 mg, 0.29 mmol) in 20 ml of abs. DMF under nitrogen atmosphere at 0°C within 15 min. To this mixture a solution of 1 (300 mg, 0.26 mmol) in 40 ml of abs. DMF was added dropwise within 15 min. After the mixture was stirred at room temp. for 18 h the solvent was removed at 40°C under reduced pressure. The resulting residual was stirred in 75 ml of acetone for 2 h. The crude product was filtered, washed with acetone and dried under reduced pressure to yield 364 mg (92%) of the title compound 3 as a light brown powder. The crude product was purified by preparative HPLC on a reverse phase column (column: Phenomenex; Luna 10 C18; solvent gradient: water /acetonitril from 0% CH3CN to 95%; flow rate: 10.5 ml/min; retention time of product: 9.3 min; detection: UV absorption 214 and 195 nm).
MP: thermal decomposition above 225°C.
MS (-p ESI, DMSO/MeOH + 10 mmol/l NH4OAc): 647.4 (75%) [M-H-Fmoc]2-, 1295.9 (15%)
[M-Fmoc]-, 1518.2 (100%) [M-H]-.
UV/Vis (MeOH/H2O 1:1) lmax [nm] (lg e): 254.6 (5.353), 289.0 (4.777), 300.0 (4.847).
1H-NMR (600 MHz): 3.25 - 3.86 (m, 42H, 7xCD-H2, 7xCD-H3, 7xCD-H4, 7xCD-H5, 7xCD-H6, 7xCD-H6'), 4.24 - 4.35 (m, 2H, 1xCD-6OH, H9), 4.42 - 4.51 (m, 7H, 5xCD-6OH, H8, H8'), 4.79 - 4.86 (m, 6H, 6xCD-H1), 4.96 (d, 3J = 3.4 Hz, 1H, 1xCD-H1), 5.65 - 5.82 (m, 14H, 7xCD-2OH, 7xCD-3OH), 7.36 (dt, 3J = 7.4 Hz, 4J = 0.9 Hz, 2H, H11, H11'), 7.43 (t, 3J = 7.4 Hz, 2H, H12, H12'), 7.77 (d, 3J = 7.4 Hz, 2H, H10, H10'), 7.91 (d, 3J = 7.4 Hz, 2H, H13, H13'), 8.03 (s, 1H, H2), 8.08 (s, 1H, H4,), 8.30 (s, 1H, H6), 8.34 (s, 1H, CD-NH), 10.01 (s, 1H, NH), 13.10 (br, 2H, COOH).
13C-NMR (150 MHz): 46.5 (+, C9), 59.4 (-, CD-C6), 59.5 (-, CD-C6), 59.7 (-, CD-C6), 59.9 (-, CD-C6), 65.7 (-, C8), 69.5 (+, CD-CH), 71.9 (+, CD-CH), 71.9 (+, CD-CH), 72.0 (+, CD-CH), 72.1 (+, CD-CH), 72.3 (+, CD-CH), 72.4 (+, CD-CH), 72.9 (+, CD-CH), 81.2 (+, CD-CH), 81.2 (+, CD-CH), 81.3 (+, CD-CH), 81.4 (+, CD-CH), 81.6 (+, CD-CH), 81.7 (+, CD-CH), 83.8 (+, CD-CH), 101.6 (+, CD-C1), 101.7 (+, CD-C1), 101.8 (+, CD-C1), 101.9 (+, CD-C1), 102.1 (+, CD-C1), 120.1 (+, C13, C13'), 121.1 (+, C6), 121.5 (+, C4), 121.9 (+, C2), 125.1 (+, C10, C10'), 127.0 (+, C11, C11'), 127.6 (+, C12, C12'), 135.5 (Cquat, C1), 139.1 (Cquat, C3), 140.7 (Cquat, C14, C14'), 143.6 (Cquat, C15, C15'), 153.3 (Cquat, C7), 165.9 (Cquat, C17), 166.8 (Cquat, C16).