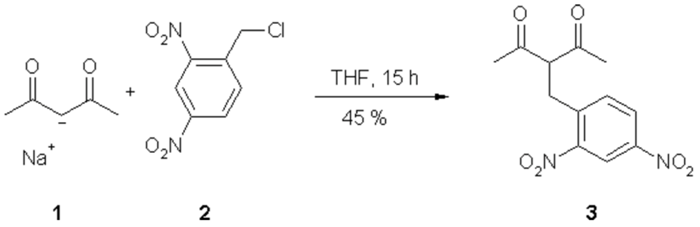
3(2,4-Dinitrobenzyl)-2,4-pentanedione
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Murakami, Y.; Nakamura, K.; Uchida, H.; Kanaoka, Y. Inorg. Chim. Acta 1968, 2(2), 133–8.
- Sample availability: available form the authors.
© 2003 MDPI. All rights reserved.
Share and Cite
Zieg, H.; Pitsch, W.; Koenig, B. 3(2,4-Dinitrobenzyl)-2,4-pentanedione. Molbank 2002, 2002, M289. https://doi.org/10.3390/M289
Zieg H, Pitsch W, Koenig B. 3(2,4-Dinitrobenzyl)-2,4-pentanedione. Molbank. 2002; 2002(1):M289. https://doi.org/10.3390/M289
Chicago/Turabian StyleZieg, Harald, Wolfgang Pitsch, and Burkhard Koenig. 2002. "3(2,4-Dinitrobenzyl)-2,4-pentanedione" Molbank 2002, no. 1: M289. https://doi.org/10.3390/M289
APA StyleZieg, H., Pitsch, W., & Koenig, B. (2002). 3(2,4-Dinitrobenzyl)-2,4-pentanedione. Molbank, 2002(1), M289. https://doi.org/10.3390/M289



