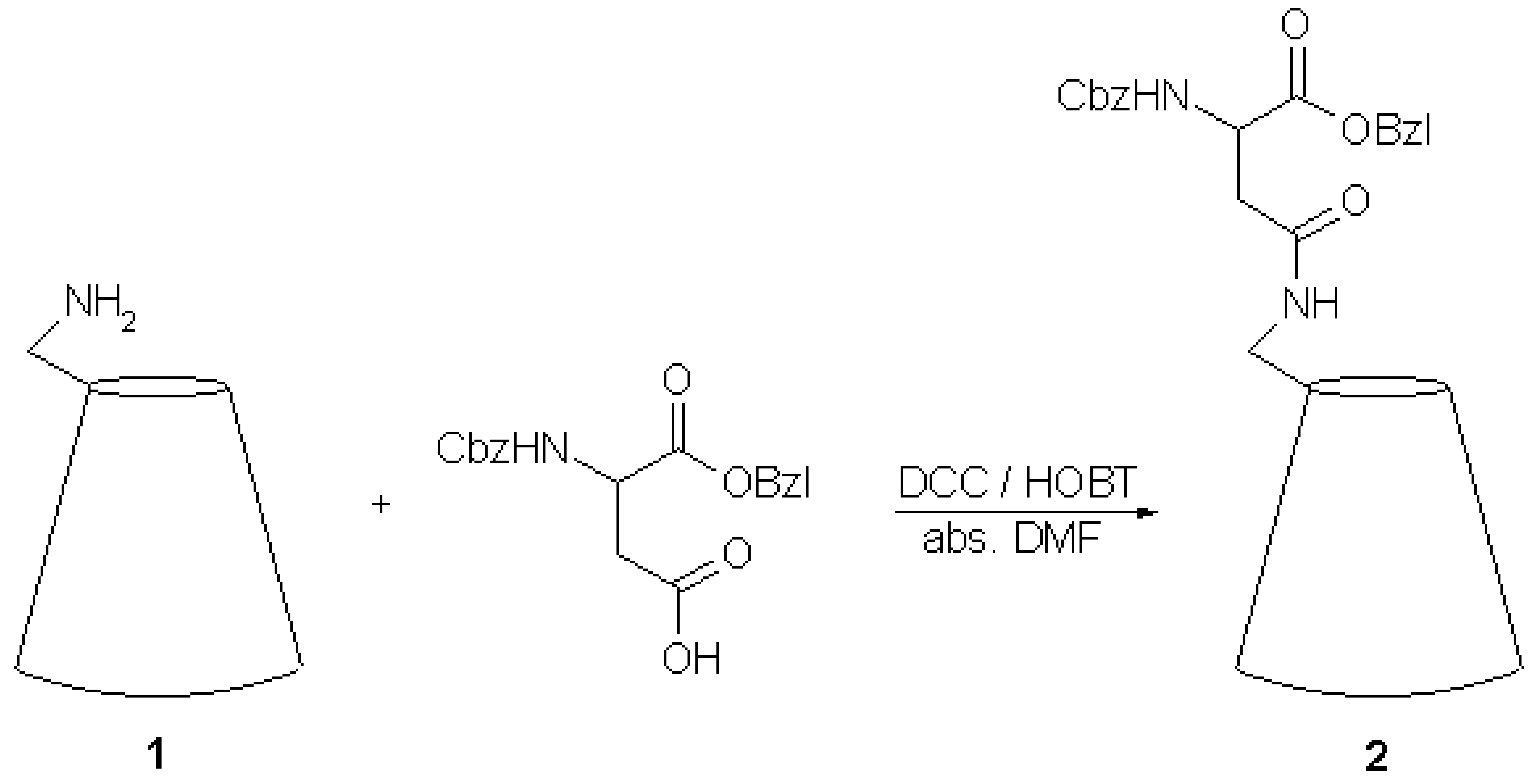
6-Amino-6-deoxy-b-cyclodextrine (1) was prepared according to the literature [1,2] in a three step synthesis. A solution of 1 (400 mg, 0.35 mmol) in 10 ml of abs. DMF was added dropwise to a solution of DCC (81 mg, 0.39 mmol), HOBT (53 mg, 0.39 mmol) and Cbz-Asp-OBzl (2) (126 mg, 0.35 mmol) in 30 ml of abs. DMF under nitrogen atmosphere at 0°C within half an hour. The reaction mixture was stirred at room temp. for 18 h, the solvent was removed at 40°C under reduced pressure. The resulting residual was stirred in 45 ml of acetone for 3 h. The crude product was filtered, washed with acetone and dried under reduced pressure to yield 514 mg (99%) of the title compound (2) as a colorless powder. The crude product was purified by preparative HPLC on a reverse phase column (column: Phenomenex; Luna 10 C18; solvent gradient: water/acetonitrile; from 0% CH3CN to 95%; flow rate: 10.5 ml/min; retention time of product: 8.2 min; detection: UV absorption 214 and 195 nm).
MP: thermal decomposition above 250°C.
MS (+p ESI, DMSO/MeOH + 10 mmol/l NH4OAc): 1382.8 (15%) [M+H - (CH2-Ph)]+, 1473.7 (100%) [M+H]+, 1495.6 (33%) [M+Na]+.
UV/Vis (MeOH/H2O 1:1) lmax [nm] (lg e): 252.1 (3.551), 257.6 (3.662), 262.9 (3.634), 268.0 (3.536), 281.5 (3.308), 306.9 (3.417).
1H-NMR (600 MHz, DMSO-D6): 2.55 - 2.71 (m, 2H, H2, H2'), 3.25 - 3.75 (m, 42H, 7xCD-H2, 7xCD-H3, 7xCD-H4, 7xCD-H5, 7xCD-H6, 7xCD-H6'), 4.36 - 4.52 (m, 7H, 6xCD-6OH, H1), 4.79 - 4.87 (m, 6H, 6xCD-H1), 5.00 - 5.13 (m, 5H, 1xCD-H1, 2xCH2-Ph), 5.61 - 5.82 (m, 14H, 7xCD-2OH, 7xCD-3OH), 7.30 - 7.39 (m, 10H, Ar-H), 7.52 (d, 3J = 8.1 Hz, 1H, NH), 7.75 (m, 1H, CD-NH).
13C-NMR (150 MHz, DMSO-D6): 36.7 (-, C2, C2', HSQC), 50.7 (+, C1, HSQC), 59.7 (-, CD-C6, HSQC), 59.8 (-, CD-C6, HSQC), 59.9 (-, CD-C6, HSQC), 59.9 (-, CD-C6, HSQC), 60.0 (-, CD-C6, HSQC), 60.1 (-, CD-C6, HSQC), 65.5 (-, CH2-Ar, HSQC), 66.1 (-, CH2-Ar, HSQC), 69.8 (+, CD-CH), 71.9 (+, CD-CH), 71.9 (+, CD-CH), 72.0 (+, CD-CH), 72.1 (+, CD-CH), 72.2 (+, CD-CH), 72.3 (+, CD-CH), 72.4 (+, CD-CH), 72.5 (+, CD-CH), 72.9 (+, CD-CH), 72.9 (+, CD-CH), 73.0 (+, CD-CH), 73.1 (+, CD-CH), 81.2 (+, CD-CH), 81.3 (+, CD-CH), 81.4 (+, CD-CH), 81.5 (+, CD-CH), 81.6 (+, CD-CH), 81..8 (+, CD-CH), 83.2 (+, CD-CH), 101.7 (+, CD-C1, HSQC), 101.8 (+, CD-C1, .HSQC), 102.0 (+, CD-C1, HSQC), 102.1 (+, CD-C1, HSQC), 102.2 (+, CD-C1, HSQC), 127.7 (+, Ar-CH, HSQC), 127.8 (+, Ar-CH, HSQC), 127.9 (+, Ar-CH, HSQC), 128.0 (+, Ar-CH, HSQC), 128.3 (+, Ar-CH, HSQC), 128.4 (+, Ar-CH, HSQC), 135.8 (Cquart, Ar-C, HMBC), 136.8 (Cquart, Ar-C, HMBC), 155.8 (Cquart, C5, HMBC), 169.0 (Cquart, C3, HMBC), 171.4 (Cquart, C4, HMBC).
Acknowledgment
We thank the Wacker-Chemie GmbH Burghausen, Germany, for their support.
References
- Matsui, Y.; Okimoto, A. Bull. Chem. Soc. Jpn. 1978, 51, 3030–3034.
- Hamasaki, K; Ikeda, H.; Nakamura, A.; Ueno, A; Toda, F.; Suzuki, I; Osa, T. J. Am. Chem. Soc. 1993, 115, 5035–5040.
© 2002 MDPI. All rights reserved.
