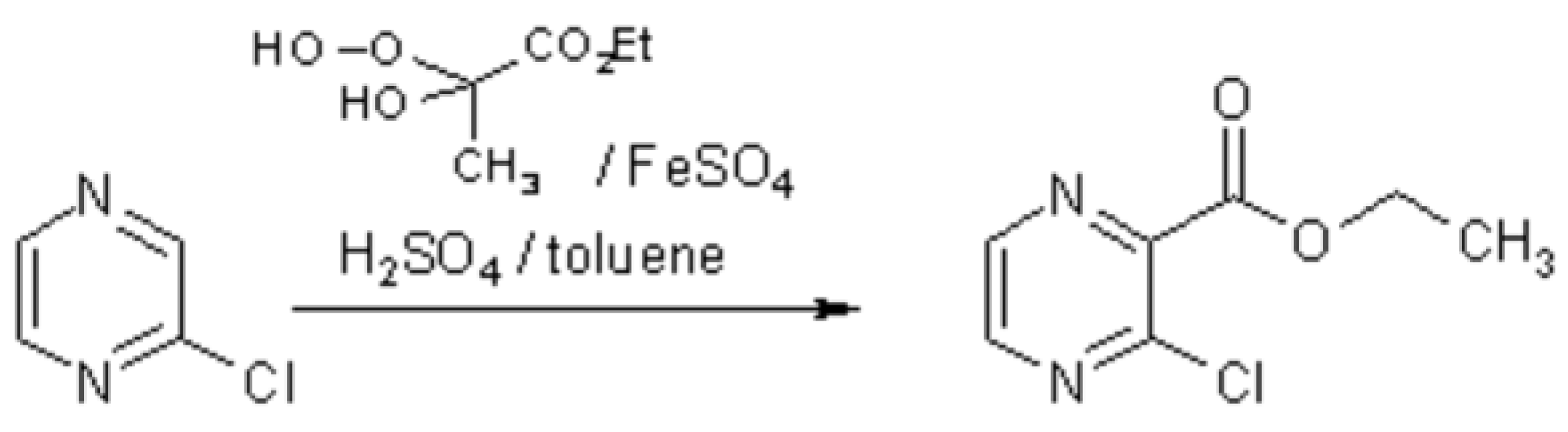
Free-radical alkoxycarbonylation of protonated pi-deficient hetarenes is a useful method for the introduction of ester functionalities into such systems [
1], especially when the reaction is carried out in a two-phase solvent system in order to suppress polysubstitution [
2]. Application of a protocol we recently used for analogous transformations of iodopyridazines into the corresponding esters, employing toluene as the organic phase [
3], was found to provide a convenient access to the new title compound which represents a useful bifunctional building block.
Thus, 3.4 g (30 mmol) of hydrogen peroxide (30%) were added dropwise between -10 and 0 °C to 5.2 g (45 mmol) of ethyl pyruvate with stirring. The viscous liquid was kept at the same temperature for 15 min, then it was added dropwise to a vigorously stirred mixture of chloropyrazine (1.14 g, 10 mmol), water (7 ml), concentrated sulfuric acid (3.0 g, 30 mmol), ferrous sulfate heptahydrate (8.3 g, 30 mmol), and toluene (30 ml) at -5 to 0 °C. Stirring was continued for another 15 min, then the mixture was poured into ice-water and it was extracted several times with dichloromethane. The combined extracts were washed with water, dried and evaporated. Excess ethyl pyruvate was removed by Kugelrohr distillation (30°C, 1 mbar). The liquid residue [
4] was purified by column chromatography on silica gel, eluting with ethyl acetate/light petroleum (1+3), followed by Kugelrohr distillation (110 °C, 1 mbar) to afford the title compound as a colorless liquid (1.04 g, 56%). Storage in a refrigerator is recommended.
1H NMR (300 MHz, CDCl3): 8.54-8.46 (AB system, J5-6 = 2.5 Hz, 2 H, H-5, H-6), 4.45 (q, J = 7.1 Hz, 2 H, CH2), 1.39 (t, J = 7.1 Hz, 3 H, CH3).
13C NMR (75 MHz, CDCl3): 163.2 (C=O), 147.2 (C-3), 145.3 (C-5), 144.7 (C-2), 141.7 (C-6), 62.6 (CH2), 13.9 (CH3).
IR (cm−1; neat): 3055, 2984, 2939, 1735 (C=O), 1550, 1515, 1445, 1383, 1301, 1181, 1147, 1068, 1013, 857.
EI-MS (70 eV): 188 (1%, M+), 186 (3, M+), 144 (15), 142 (45), 141 (25), 116 (32), 114 (100), 113 (39), 86 (27), 79 (33), 62 (22), 52 (49), 51 (33).
Anal. calc. for C7H7ClN2O2 (186.60): C 45.06, H 3.78, N 15.01; found: C 45.4, H 3.6, N 14.6.