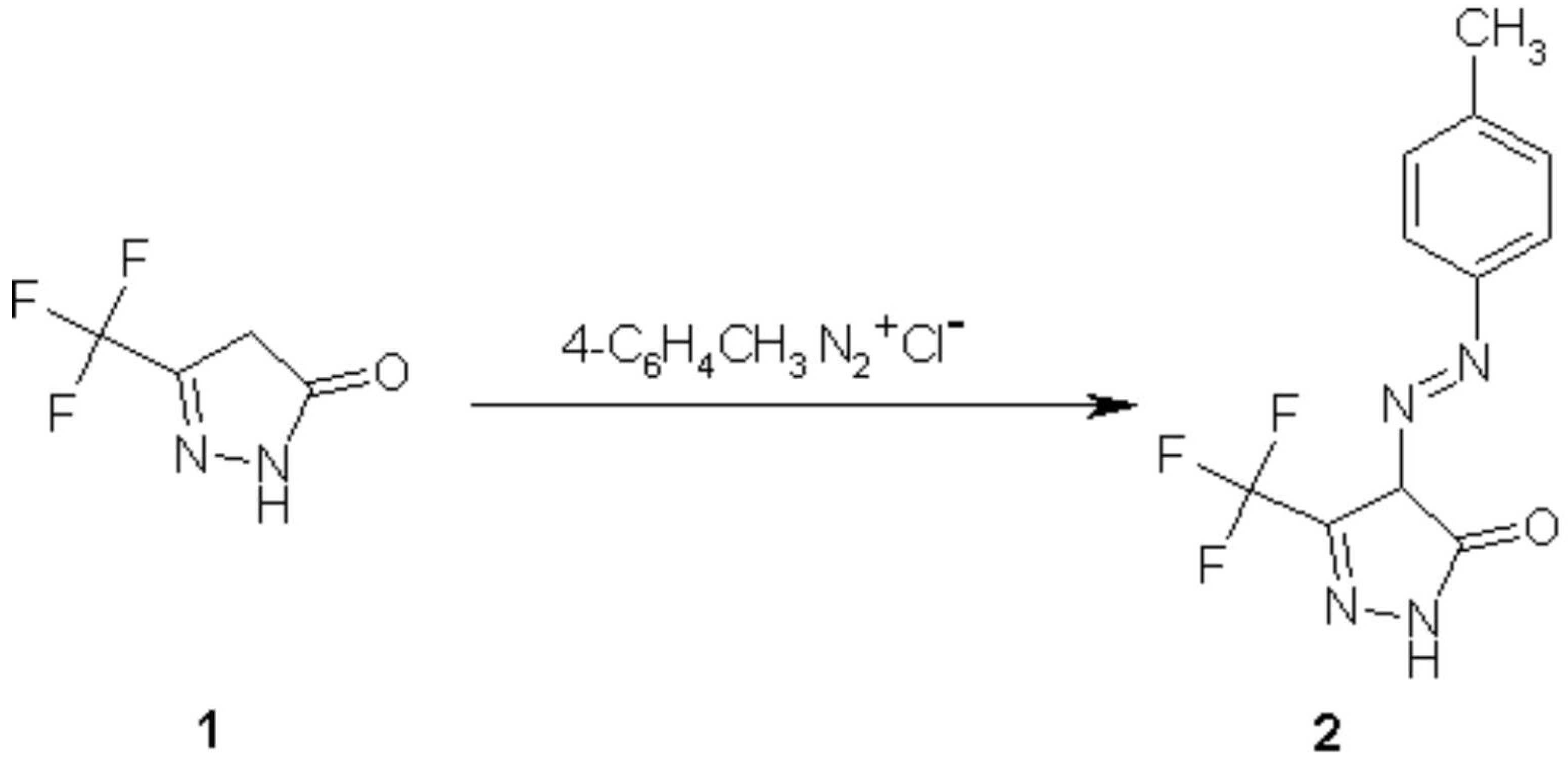
4-p-Tolylazo-5-trifluoromethyl-2,4-dihydropyrazol-3-one
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Zohdi, H. F.; Elghandour, A. H. H.; Rateb, N. M.; Sallam, M. M. M. J. J. Chem. Res. 1992. (S) 396, (M) 3015.
- Zohdi, H. F.; Rateb, N. M. Molecules 2001, 6, M261.
- Sample Availability: Available from the authors and from MDPI
© 2003 MDPI. All rights reserved.
Share and Cite
Zohdi, H.F.; Rateb, N.M. 4-p-Tolylazo-5-trifluoromethyl-2,4-dihydropyrazol-3-one. Molbank 2003, 2003, M305. https://doi.org/10.3390/M305
Zohdi HF, Rateb NM. 4-p-Tolylazo-5-trifluoromethyl-2,4-dihydropyrazol-3-one. Molbank. 2003; 2003(1):M305. https://doi.org/10.3390/M305
Chicago/Turabian StyleZohdi, Hussein F., and Nora M. Rateb. 2003. "4-p-Tolylazo-5-trifluoromethyl-2,4-dihydropyrazol-3-one" Molbank 2003, no. 1: M305. https://doi.org/10.3390/M305
APA StyleZohdi, H. F., & Rateb, N. M. (2003). 4-p-Tolylazo-5-trifluoromethyl-2,4-dihydropyrazol-3-one. Molbank, 2003(1), M305. https://doi.org/10.3390/M305



