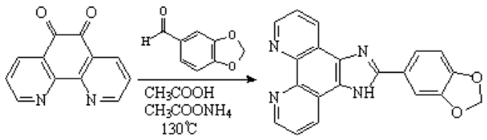
2’-(3’’,4’’-Methylenedioxyphenyl)imidazo[4’,5’-f]1,10-phenanthroline
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgement
References
- Paw, W.; Eisenberg, R. Inorg. Chem. 1997, 36, 2287.
© 2003 MDPI. All rights reserved.
Share and Cite
Wang, Z.-M.; Hao, Y.-M.; Liu, J.-Z.; Ji, L.-N. 2’-(3’’,4’’-Methylenedioxyphenyl)imidazo[4’,5’-f]1,10-phenanthroline. Molbank 2002, 2002, M286. https://doi.org/10.3390/M286
Wang Z-M, Hao Y-M, Liu J-Z, Ji L-N. 2’-(3’’,4’’-Methylenedioxyphenyl)imidazo[4’,5’-f]1,10-phenanthroline. Molbank. 2002; 2002(1):M286. https://doi.org/10.3390/M286
Chicago/Turabian StyleWang, Zhong-Ming, Yong-Mei Hao, Jian-Zhong Liu, and Lian-Nian Ji. 2002. "2’-(3’’,4’’-Methylenedioxyphenyl)imidazo[4’,5’-f]1,10-phenanthroline" Molbank 2002, no. 1: M286. https://doi.org/10.3390/M286
APA StyleWang, Z.-M., Hao, Y.-M., Liu, J.-Z., & Ji, L.-N. (2002). 2’-(3’’,4’’-Methylenedioxyphenyl)imidazo[4’,5’-f]1,10-phenanthroline. Molbank, 2002(1), M286. https://doi.org/10.3390/M286



