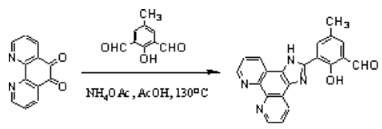
2-(2-Hydroxy-5-methyl-3-formylphenyl)-imidazo[4,5-f][1,10]-phenanthroline
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgement
References
- Yamada, M.; Tanaka, Y.; Yoshimato, Y.; Kuroda, S.; Shimao, I. Bull. Chem. Soc. Jpn. 1992, 65, 1006. [CrossRef]
© 2003 MDPI. All rights reserved.
Share and Cite
Chao, H.; Jiang, C.-W.; Hong, X.-L.; Ji, L.-N. 2-(2-Hydroxy-5-methyl-3-formylphenyl)-imidazo[4,5-f][1,10]-phenanthroline. Molbank 2002, 2002, M285. https://doi.org/10.3390/M285
Chao H, Jiang C-W, Hong X-L, Ji L-N. 2-(2-Hydroxy-5-methyl-3-formylphenyl)-imidazo[4,5-f][1,10]-phenanthroline. Molbank. 2002; 2002(1):M285. https://doi.org/10.3390/M285
Chicago/Turabian StyleChao, Hui, Cai-Wu Jiang, Xian-Lan Hong, and Liang-Nian Ji. 2002. "2-(2-Hydroxy-5-methyl-3-formylphenyl)-imidazo[4,5-f][1,10]-phenanthroline" Molbank 2002, no. 1: M285. https://doi.org/10.3390/M285
APA StyleChao, H., Jiang, C.-W., Hong, X.-L., & Ji, L.-N. (2002). 2-(2-Hydroxy-5-methyl-3-formylphenyl)-imidazo[4,5-f][1,10]-phenanthroline. Molbank, 2002(1), M285. https://doi.org/10.3390/M285



