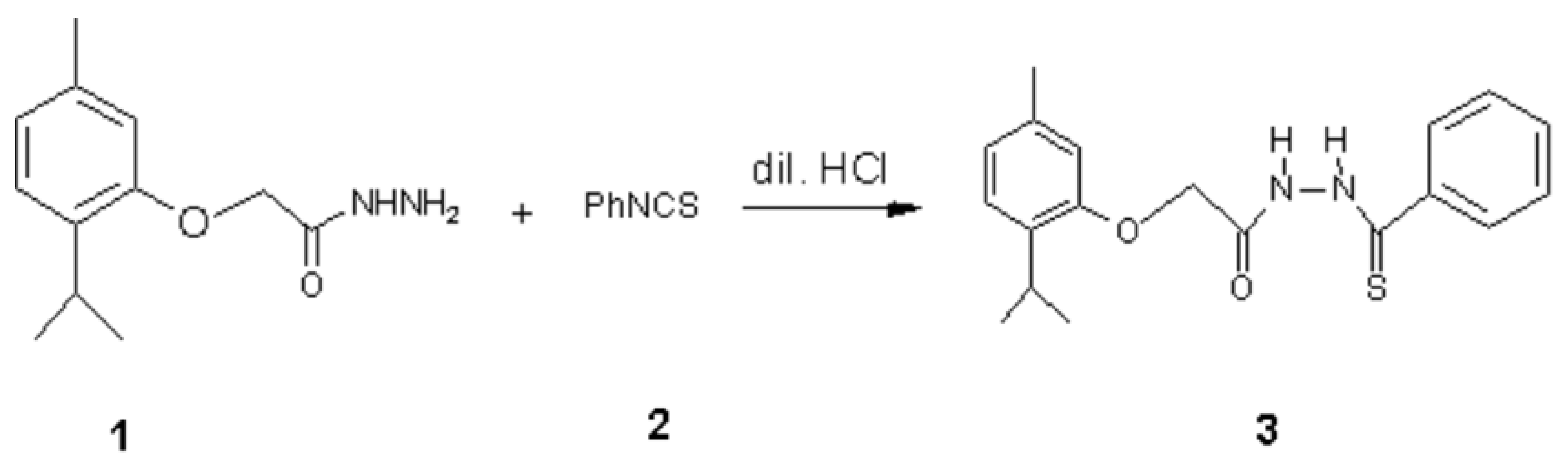
2-[(2-Isopropyl-5-methylphenoxy)acetyl]-N-phenylhydrazine Carbothioamide
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Trivedi, S.; Kubavate, H.; Parekh, H. Indian J. Chem. 1994, 33, 295.
- Vashi, B. S.; Mehta, S.; Shah, V. H. Indian J. Chem. 1996, 35, 11.
© 2003 MDPI. All rights reserved.
Share and Cite
Asiri, A.M. 2-[(2-Isopropyl-5-methylphenoxy)acetyl]-N-phenylhydrazine Carbothioamide. Molbank 2002, 2002, M279. https://doi.org/10.3390/M279
Asiri AM. 2-[(2-Isopropyl-5-methylphenoxy)acetyl]-N-phenylhydrazine Carbothioamide. Molbank. 2002; 2002(1):M279. https://doi.org/10.3390/M279
Chicago/Turabian StyleAsiri, Abdullah Mohamed. 2002. "2-[(2-Isopropyl-5-methylphenoxy)acetyl]-N-phenylhydrazine Carbothioamide" Molbank 2002, no. 1: M279. https://doi.org/10.3390/M279
APA StyleAsiri, A. M. (2002). 2-[(2-Isopropyl-5-methylphenoxy)acetyl]-N-phenylhydrazine Carbothioamide. Molbank, 2002(1), M279. https://doi.org/10.3390/M279



