Application of Electron Paramagnetic Resonance Spectroscopy for Validation of the Novel (AN+DN) Solvent Polarity Scale
Abstract
:1. Introduction
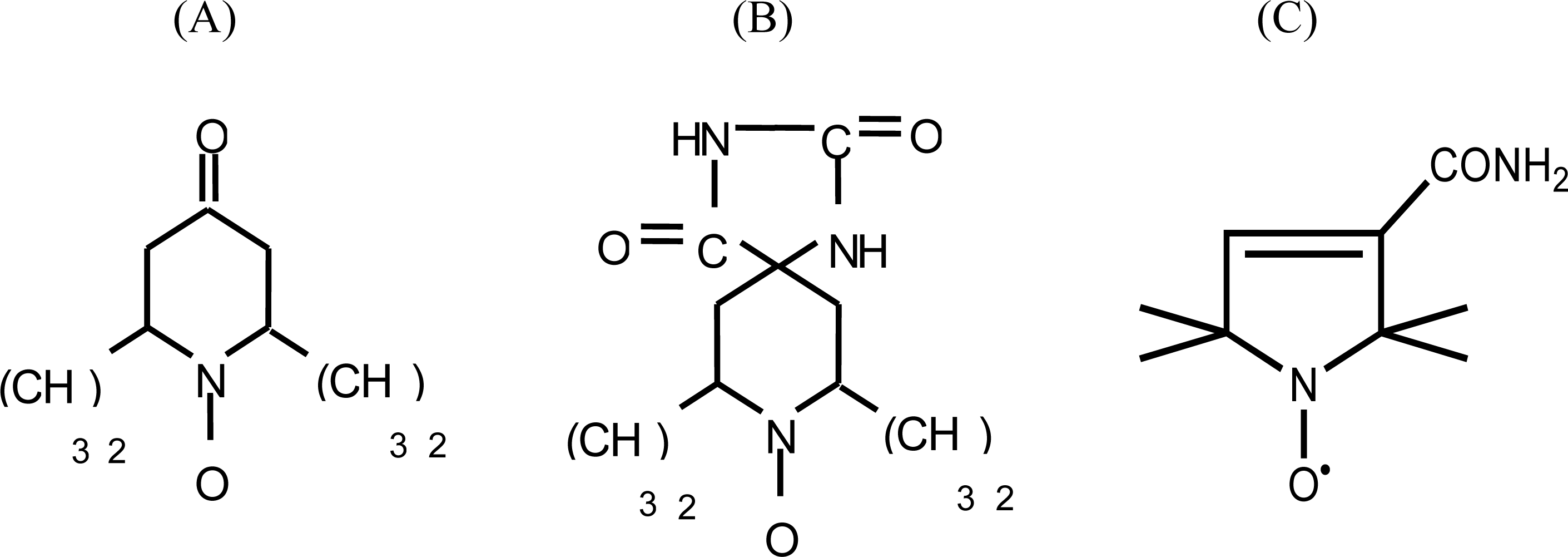
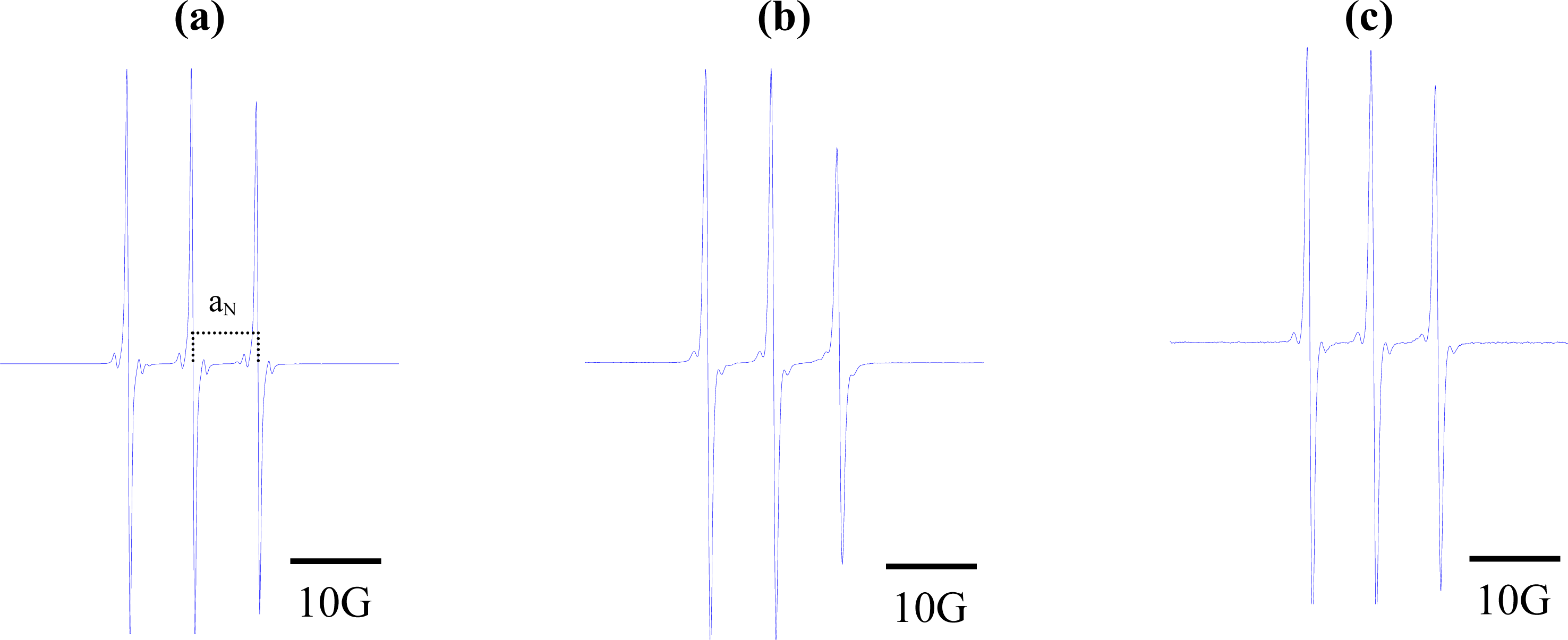
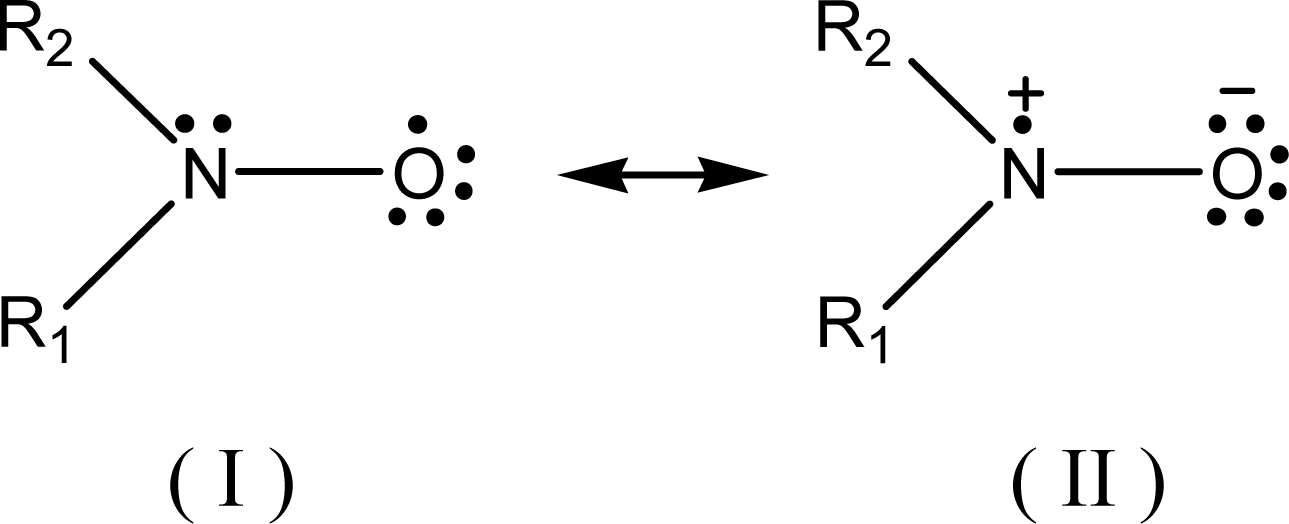
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Methods
Acknowledgments
References
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem Rev 1994, 94, 2319–2358. [Google Scholar]
- Katritzky, AR; Fara, DC; Yang, HF; Tamm, K; Tamm, K; Karelson, M. Quantitative Measures of Solvent Polarity. Chem Rev 2004, 104, 175–198. [Google Scholar]
- Cilli, EM; Oliveira, E; Marchetto, R; Nakaie, CR. Correlation between Solvation of Peptide-Resins and Solvent Properties. J Org Chem 1996, 61, 8992–9000. [Google Scholar]
- Malavolta, L; Oliveira, E; Cilli, EM; Nakaie, CR. Solvation of Polymers as Model for Solvent Effect Investigation: Proposition of a Novel Polarity Scale. Tetrahedron 2002, 58, 4383–4394. [Google Scholar]
- Gutmann, V. The Donor-acceptor Approach to Molecular Interactions; Plenum: New York, USA, 1978. [Google Scholar]
- Oliveira, E; Cilli, EM; Miranda, A; Jubilut, GN; Albericio, A; Andreu, A; Paiva, ACM; Schreier, S; Nakaie, CR. Monitoring the Chemical Assembly of a Transmembrane Bradykinin Receptor Fragment: Correlation between Resin Solvation, Peptide Chain Mobility and Rate of Coupling. Eur J Org Chem 2002, 21, 3686–3694. [Google Scholar]
- Barany, G; Merrifield, RB. The Peptides: Analysis, Synthesis and Biology; Gross, E, Meienhofer, J, Eds.; Academic Press: New York, USA, 1980; pp. 1–284. [Google Scholar]
- Kates, SA; Albericio, F. Solid Phase Synthesis: A Practical Guide; Dekker, M, Ed.; New York, USA, 2000; pp. 275–330. [Google Scholar]
- Fields, GB; Noble, RL. Solid-Phase Peptide-Synthesis Utilizing 9-Fluorenylmethoxycarbonyl Amino-Acids. Int J Pept Prot Res 1990, 35, 161–214. [Google Scholar]
- Cilli, EM; Marchetto, R; Schreier, S; Nakaie, CR. Correlation between the Mobility of Spin Labeled Peptide Chains and Resin Solvation: An Approach to Optimize the Synthesis of Aggregating Sequences. J Org Chem 1999, 64, 9118–9123. [Google Scholar]
- Marchetto, R; Cilli, EM; Jubilut, GN; Schreier, S; Nakaie, CR. Determination of Site-Site Distance and Site Concentration within Polymer Beads: A Combined Swelling-Electron Paramagnetic Resonance Study. J Org Chem 2005, 70, 4561–4568. [Google Scholar]
- Nakaie, CR; Malavolta, L; Schreier, S; Trovatti, E; Marchetto, R. Direct Electron Paramagnetic Resonance Monitoring of the Peptide Synthesis Coupling Reaction in Polymeric Support. Polymer 2006, 47, 4531–4536. [Google Scholar]
- Malavolta, L; Nakaie, CR. Peptide Dissociation in Solution or Bound to a Polymer: Comparative Solvent Effect. Tetrahedron 2004, 60, 9417–9424. [Google Scholar]
- Malavolta, L; Pinto, MRS; Cuvero, JH; Nakaie, CR. Interpretation of the Dissolution of Insoluble Peptide Sequences Base on the Acid-Base Properties of Solvent. Protein Sci 2006, 15, 1476–1488. [Google Scholar]
- Lynn, DG; Meredith, SC. Model Peptides and the Physicochemical Approach to β-Amyloids. J Struct Biol 2000, 130, 153–173. [Google Scholar]
- De-Felice, FG; Vieira, MNN; Saraiva, LM; Figueroa-Villar, JD; Garcia-Abreu, J; Liu, R; Chang, L; Klein, WL; Ferreira, ST. Targeting the Neurotoxic Species in Alzheimer'sDisease: Inhibitors of Aβ Oligomerization. Faseb J 2004, 18, 1366–1372. [Google Scholar]
- Brière, R; Lemaire, H; Rassat, A. Nitroxydes XV: Synthese et Etude de Radicaux Libres Stables Pipéridiniques et Pyrrolidinique. Bull Soc Chim Fr 1965, 3273–3283. [Google Scholar]
- Marsh, D. Polarity Contributions to Hyperfine Splittings of Hydrogen-Bonded Nitroxides – The Microenvironment of Spin Labels. J Magn Reson 2002, 157, 114–118. [Google Scholar]
- Plato, M; Steinhoff, HJ; Wegener, C; Torring, JT; Savitsky, A; Möbius, K. Molecular Orbital Study of Polarity and Hydrogen Bonding Effects on the G and Hyperfine Tensors of Site Directed NO Spin Labeled Bacteriorhodopsin. Mol Phys 2002, 100, 3711–3721. [Google Scholar]
- Marsh, D; Toniolo, C. Polarity Dependence of EPR Parameters for TOAC and MTSSL Spin Labels: Correlation with DOXYL Spin Labels for Membrane Studies. J Magn Res 2008, 190, 211–221. [Google Scholar]
- Rassat, A; Rey, P. Nitroxydes. 23. Préparation Dàminoacides Radicalaires et de Leurs Sels Complexes. Bull Soc Chim Fr 1967, 815–817. [Google Scholar]
- Rozantsev, EG. Free Nitroxyl Radicals; Plenum Press: New York, USA, 1970. [Google Scholar]
- Nakaie, CR; Goissis, G; Schreier, S; Paiva, ACM. pH Dependence of ESR Spectra of Nitroxide Containing Ionizable Groups. Braz J Med Biol Res 1981, 14, 173–180. [Google Scholar]
- Marchetto, R; Schreier, S; Nakaie, CR. A Novel Spin-Labeled Amino Acid Derivative for Use in Peptide Synthesis: (9-Fluorenylmethyloxycarbonyl) 2,2,6,6-Tetramethylpiperidine-N-Oxyl-4-Amino-4-Carboxylic Acid. J Am Chem Soc 1993, 115, 11042–11043. [Google Scholar]
- Tominaga, M; Barbosa, SR; Poletti, EF; Zukerman-Schpector, J; Marchetto, R; Schreier, S; Paiva, ACM; Nakaie, CR. Fmoc-POAC: [(9-Fluorenylmethyloxycarbonyl)-2,2,5,5-Tetramethylpyrrolidine-N-Oxyl-3-Amino-4-Carboxylic acid]: A Novel Protected Spin Labeled Beta-amino Acid for Peptide and Protein Chemistry. Chem Pharm Bull 2001, 49, 1027–1029. [Google Scholar]
- Dimroth, K; Bohlman, F; Reichardt, C; Siepmann, T. Uber Pyridinium-N-Phenol-Betaine Und Ihre Verwendung Zur Charakterisierung Der Polaritat Von Losungsmitteln. Liebigs Ann Chem 1963, 661, 1–37. [Google Scholar]
- Barton, AFM. Solubility Parameters. Chem Rev 1975, 75, 731–753. [Google Scholar]
- Parker, AJ. Protic-Dipolar Aprotic Solvent Effects on Rates of Bimolecular Reactions. Chem Rev 1969, 69, 1–32. [Google Scholar]
- Chastrette, M; Carretto, J. Statistical Study of Solvent Effects-II Analysis of Some Empirical Parameters of Solvent Polarity. Tetrahedron 1982, 38, 1615–1618. [Google Scholar]
- Berliner, JL; Reuben, J. Spin Labeling Theory and Applications; Plenum Press: New York, 1989. [Google Scholar]
- Griffith, OH; Dehlinger, PJ; Van, SP. Shape or the Hydrophobic Barrier of Phospholipids Bilayers (Evidence for Water Penetration in Biological Membranes). J Memb Biol 1974, 15, 159–192. [Google Scholar]
- Subczynski, WK; Wisniewska, A; Yin, JJ; Hyde, JS; Kusumi, A. Hydrophobic Barriers of Lipid Bilayer Membranes Formed by Reduction of Water Penetration by Alkyl Chain Unsaturation and Cholesterol. Biochemistry 1994, 33, 7670–7681. [Google Scholar]
- Marsh, D. Reaction Fields and Solvent Dependence of the EPR Parameters of Nitroxides: the Microenvironment of Spin Labels. J Mag Res 2008, 190, 60–67. [Google Scholar]
- Stewart, JJJ. Optimization of Parameters for Semiempirical Methods. II. Applications. J Comput Chem 1989, 10, 221–264. [Google Scholar]
- Un, S; Atta, M; Fontecave, M; Rutherford, AW. G-values as a Probe for the Local Protein Environment: High-field EPR of Tyrosyl Radicals in Ribonucleotide Reductase and Photosystem II. J Am Chem Soc 1995, 117, 10713–10719. [Google Scholar]
- Engström, M; Owenius, R; Vahtras, O. Ab Initial G-tensor Calculations of Hydrogen Bond Effects on a Nitroxide Spin Label. Chem Phys Lett 2001, 338, 407–413. [Google Scholar]
- Reichardt, C. Empirical Parameters of the Polarity of Solvents. Angew Chem 1965, 77, 30–40. [Google Scholar]
- Müller, P. Glossary of Terms Used in Physical Organic Chemistry (IUPAC Recommendations 1994). Pure Appl Chem 1994, 66, 1077–1184. [Google Scholar]
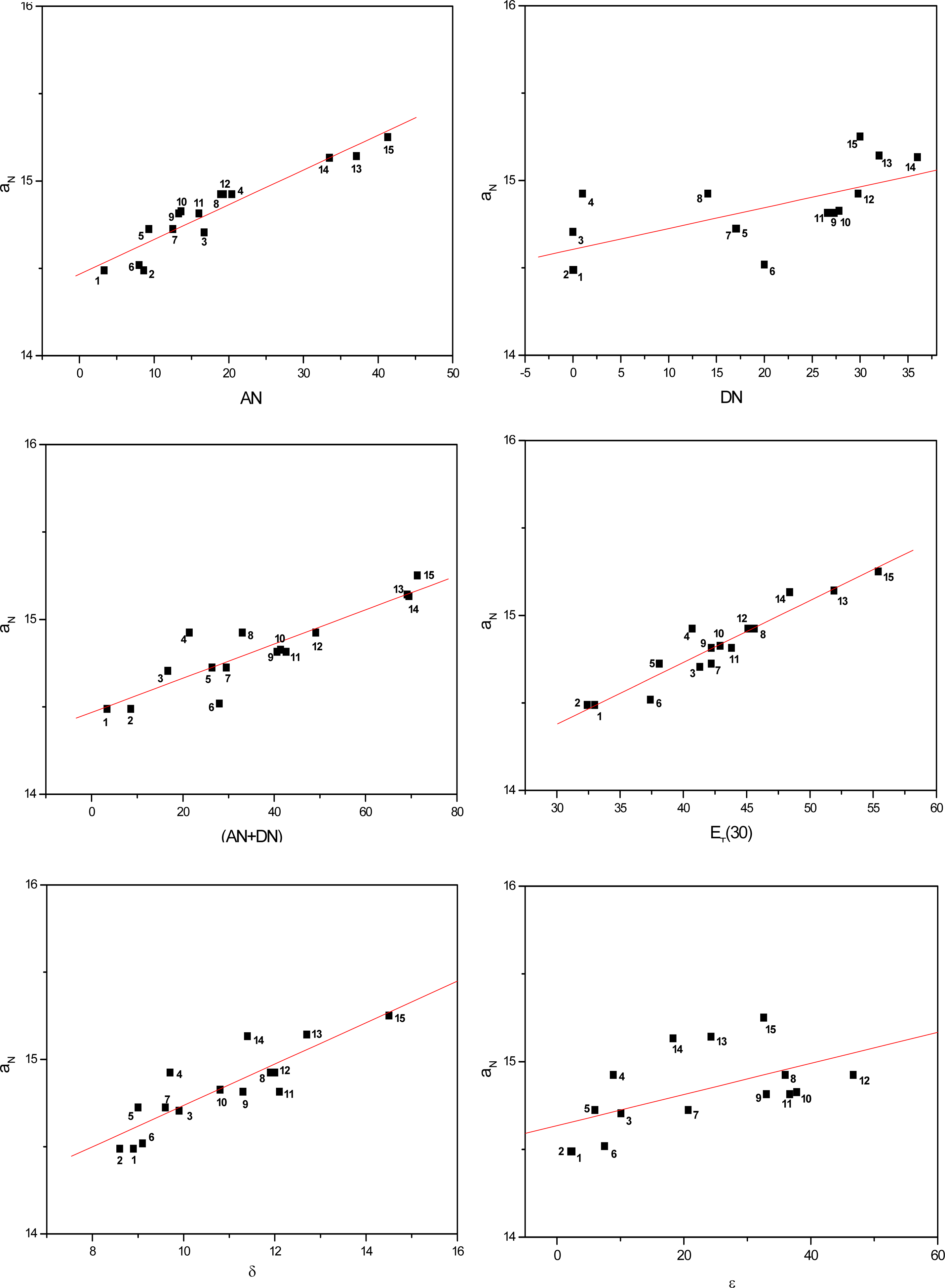
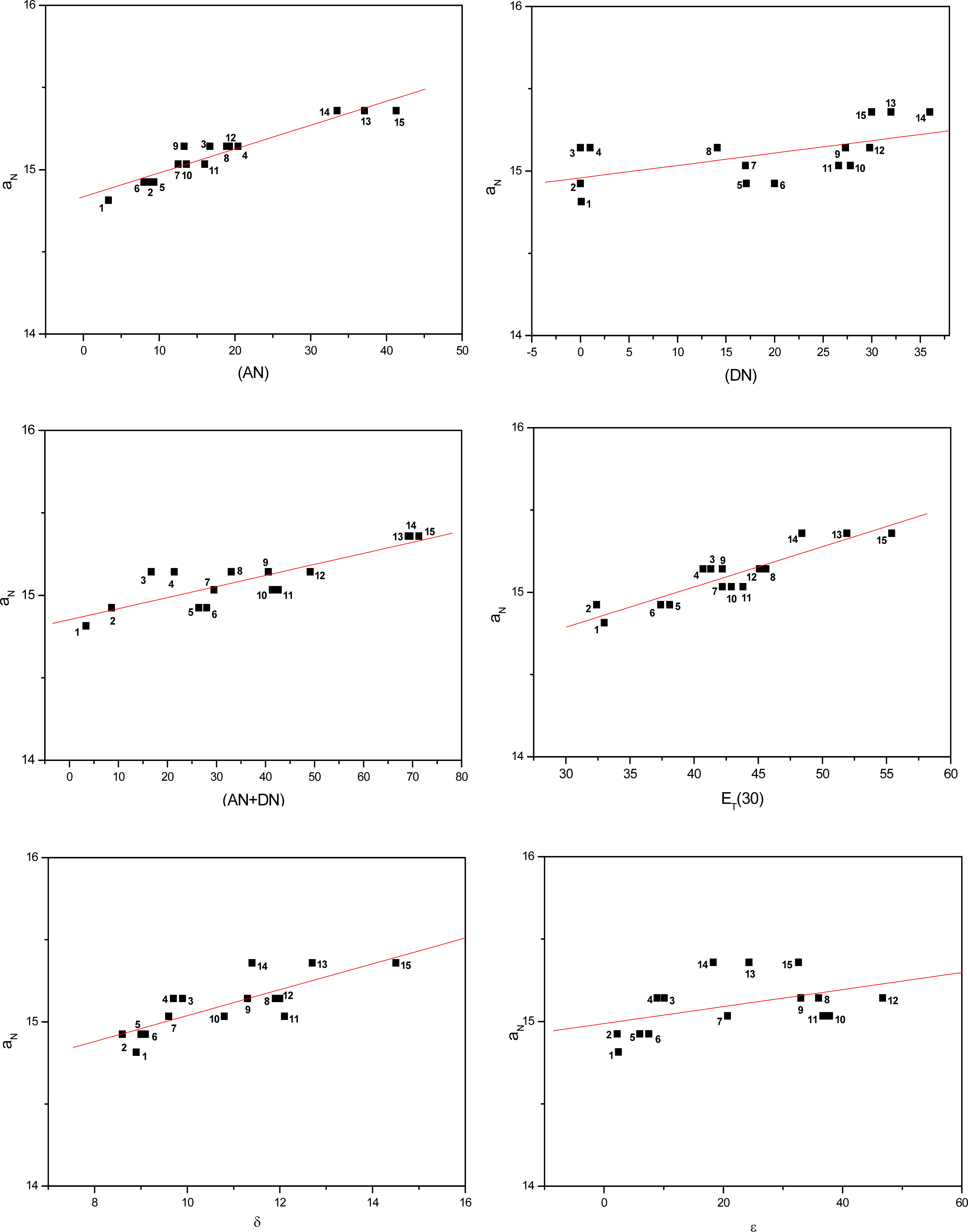
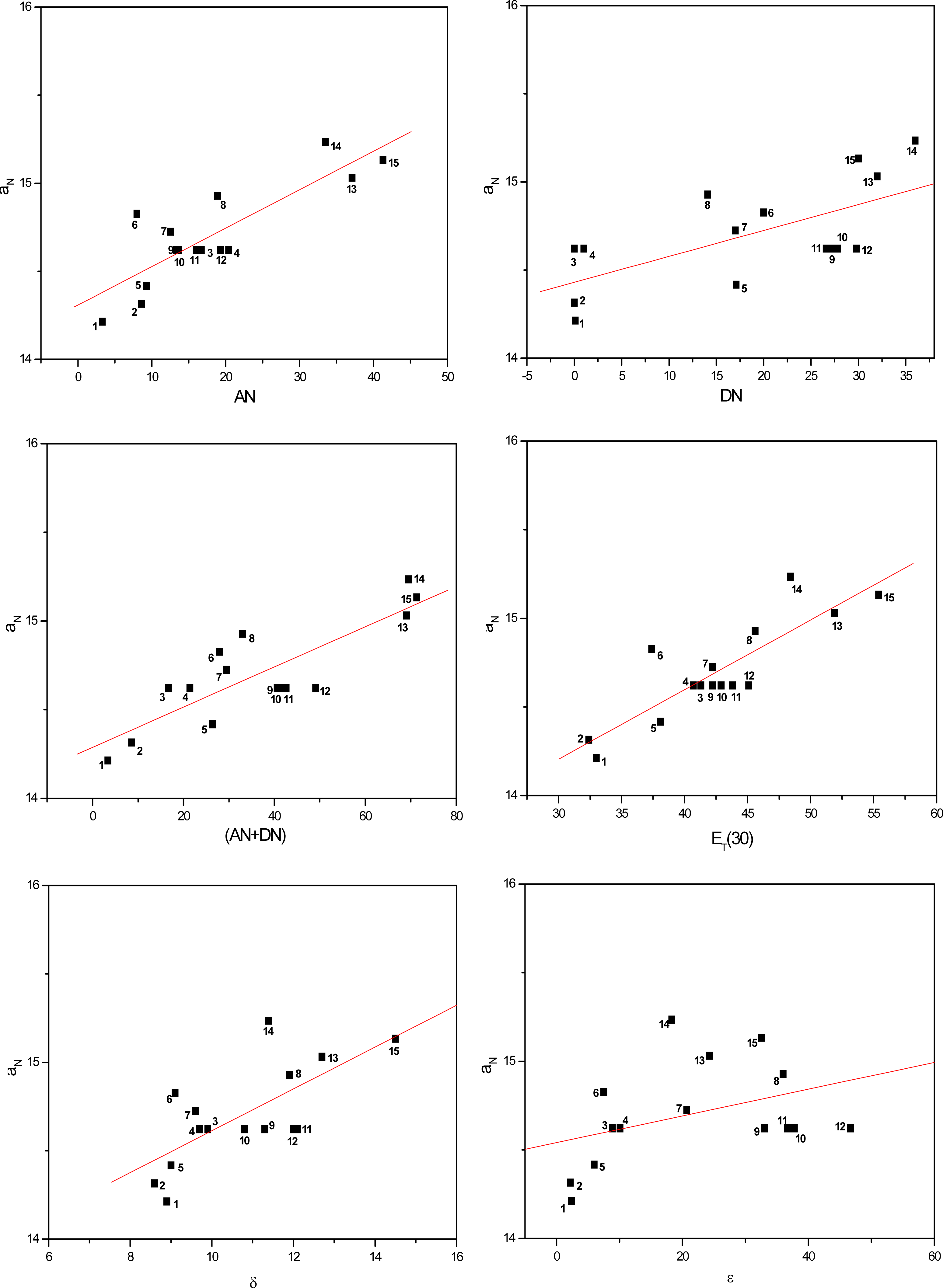
| Solvent | ANa | DNa | (AN+DN)b | ET(30)c (kcal/mol) | δd (cal/mL)1/2 | εe | |
|---|---|---|---|---|---|---|---|
| 1 | Toluene | 3.3 | 0.1 | 3.4 | 33.0 | 8.9 | 2.4 |
| 2 | Carbon tetrachloride | 8.6 | 0.0 | 8.6 | 32.4 | 8.6 | 2.2 |
| 3 | 1,2-Dichloroethane | 16.7 | 0.0 | 16.7 | 41.3 | 9.9 | 10.1 |
| 4 | DCM | 20.4 | 1.0 | 21.4 | 40.7 | 9.7 | 8.9 |
| 5 | Ethyl acetate | 9.3 | 17.1 | 26.4 | 38.1 | 9.0 | 6.0 |
| 6 | THF | 8.0 | 20.0 | 28.0 | 37.4 | 9.1 | 7.5 |
| 7 | Acetone | 12.5 | 17.0 | 29.5 | 42.2 | 9.6 | 20.7 |
| 8 | Acetonitrile | 18.9 | 14.1 | 33.0 | 45.6 | 11.9 | 36.0 |
| 9 | NMP | 13.3 | 27.3 | 40.6 | 42.2 | 11.3 | 33.0 |
| 10 | Dimethylacetamide | 13.6 | 27.8 | 41.4 | 42.9 | 10.8 | 37.8 |
| 11 | DMF | 16.0 | 26.6 | 42.6 | 43.8 | 12.1 | 36.7 |
| 12 | DMSO | 19.3 | 29.8 | 49.1 | 45.1 | 12.0 | 46.7 |
| 13 | EtOH | 37.1 | 32.0 | 69.1 | 51.9 | 12.7 | 24.3 |
| 14 | Propanol | 33.5 | 36.0 | 69.5 | 48.4 | 11.4 | 18.3 |
| 15 | MeOH | 41.3 | 30.0 | 71.3 | 55.4 | 14.5 | 32.6 |
| Parameter | Linear regression coefficient (r)
| |||
|---|---|---|---|---|
| A | B | C | (x̄)a | |
| AN | 0.94 | 0.95 | 0.84 | 0.91 |
| DN | 0.66 | 0.58 | 0.66 | 0.63 |
| (AN+DN) | 0.89 | 0.85 | 0.84 | 0.86 |
| ET(30) | 0.95 | 0.91 | 0.86 | 0,91 |
| δ | 0.86 | 0.79 | 0.70 | 0.78 |
| ε | 0.56 | 0.45 | 0.39 | 0.47 |
Share and Cite
Malavolta, L.; Poletti, E.F.; Silva, E.H.; Schreier, S.; Nakaie, C.R. Application of Electron Paramagnetic Resonance Spectroscopy for Validation of the Novel (AN+DN) Solvent Polarity Scale. Int. J. Mol. Sci. 2008, 9, 1321-1332. https://doi.org/10.3390/ijms9071321
Malavolta L, Poletti EF, Silva EH, Schreier S, Nakaie CR. Application of Electron Paramagnetic Resonance Spectroscopy for Validation of the Novel (AN+DN) Solvent Polarity Scale. International Journal of Molecular Sciences. 2008; 9(7):1321-1332. https://doi.org/10.3390/ijms9071321
Chicago/Turabian StyleMalavolta, Luciana, Erick F. Poletti, Elias H. Silva, Shirley Schreier, and Clovis R. Nakaie. 2008. "Application of Electron Paramagnetic Resonance Spectroscopy for Validation of the Novel (AN+DN) Solvent Polarity Scale" International Journal of Molecular Sciences 9, no. 7: 1321-1332. https://doi.org/10.3390/ijms9071321
APA StyleMalavolta, L., Poletti, E. F., Silva, E. H., Schreier, S., & Nakaie, C. R. (2008). Application of Electron Paramagnetic Resonance Spectroscopy for Validation of the Novel (AN+DN) Solvent Polarity Scale. International Journal of Molecular Sciences, 9(7), 1321-1332. https://doi.org/10.3390/ijms9071321




