Supported Phospholipid Bilayer Interaction with Components Found in Typical Room-Temperature Ionic Liquids – a QCM-D and AFM Study
Abstract
1. Introduction
2. Results and Discussion
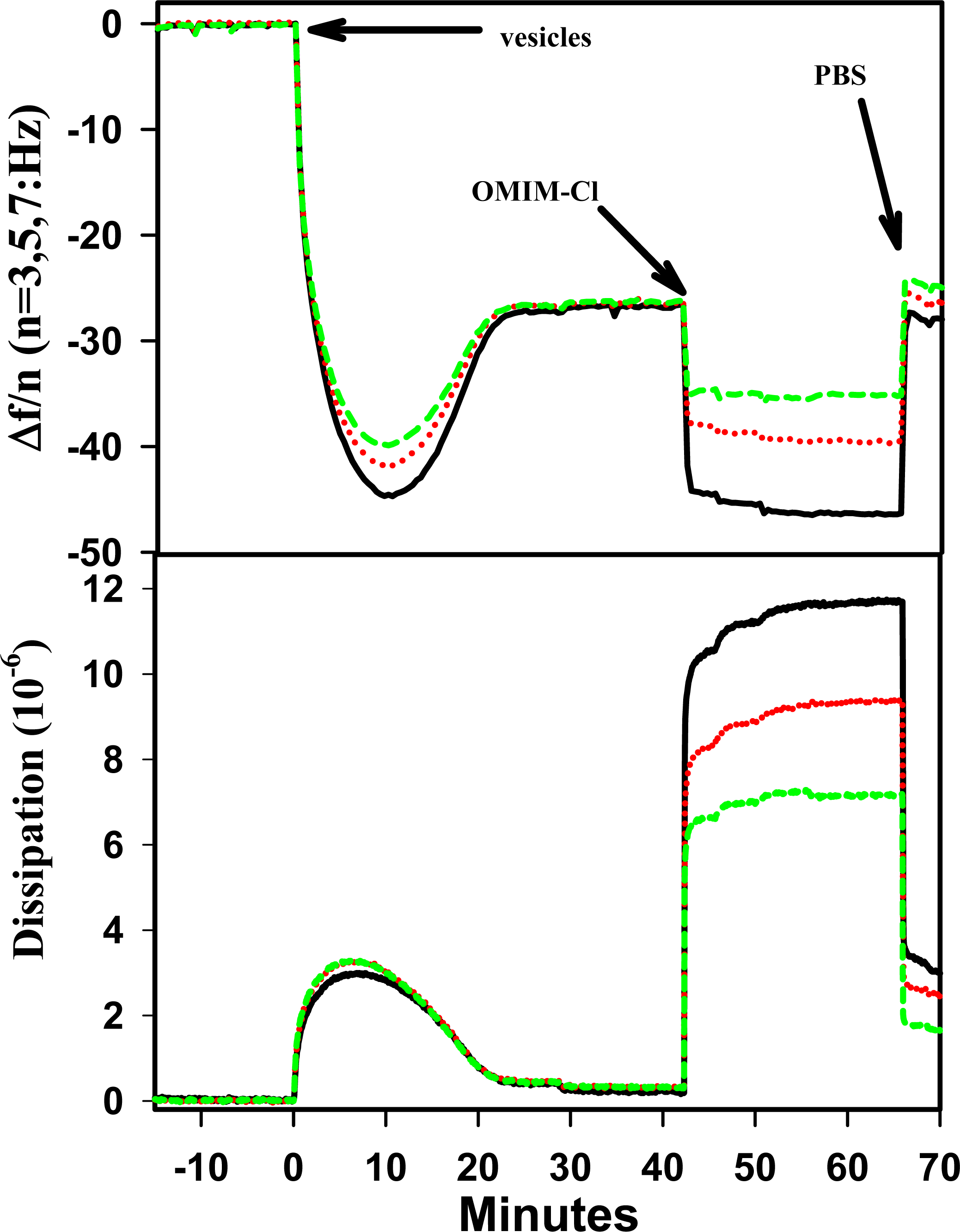
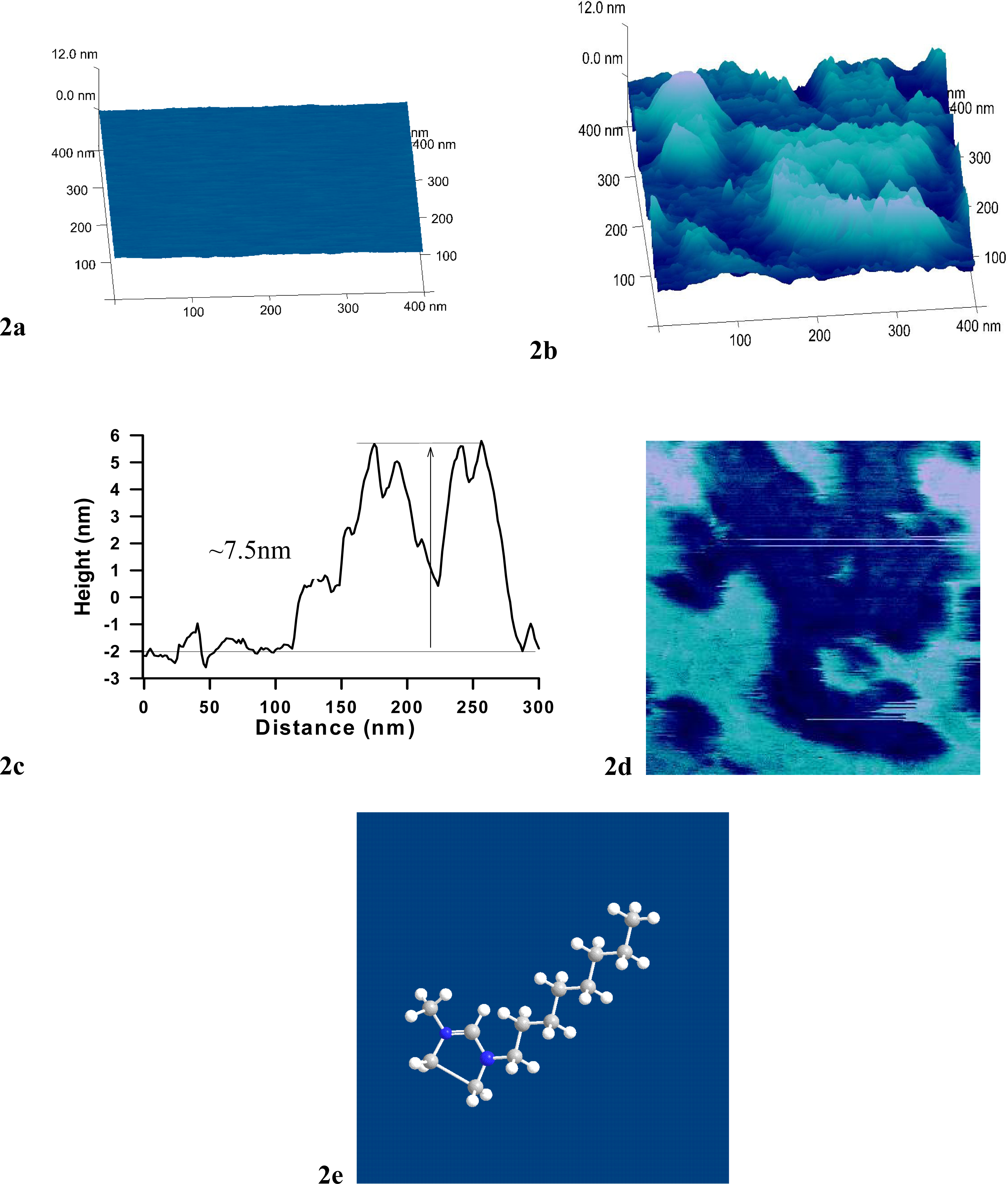
2.1. Effect of 1-Octyl-3-Methyl imidazolium (OMIM+) on 1,2-dielaidoylphosphatidylcholine (DEPC) Supported Phospholipid Bilayer (SPB)
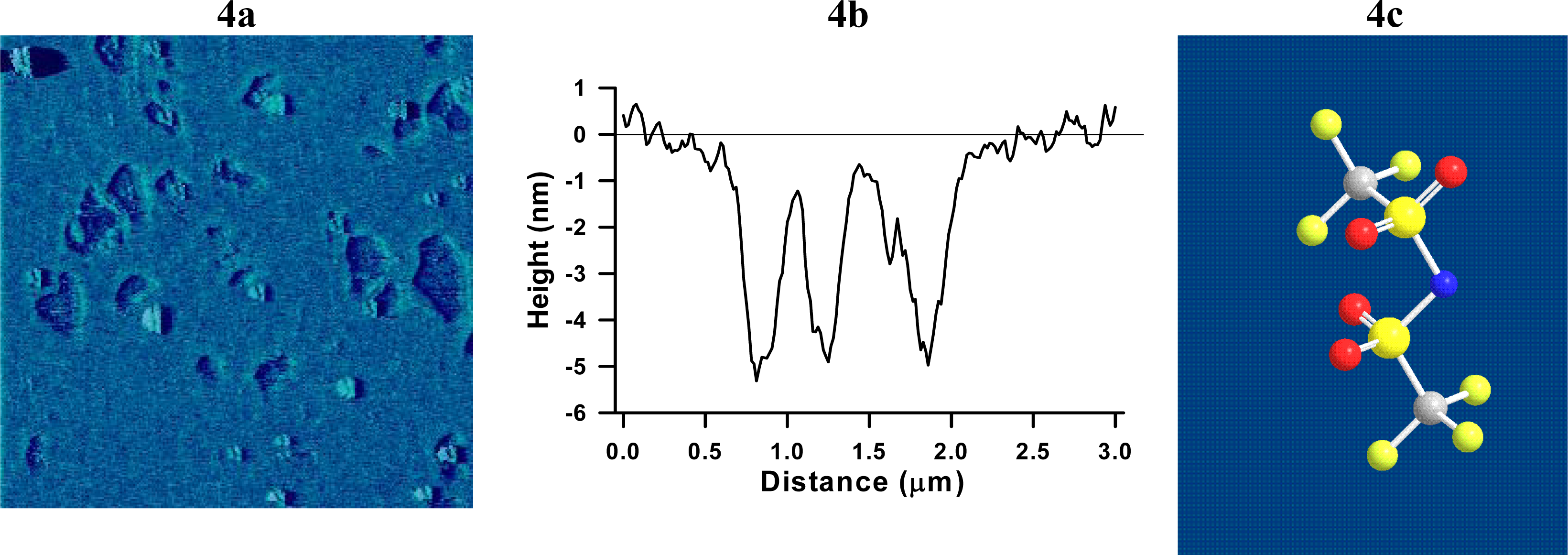
2.2 Interaction of Tf2N− with a SPB
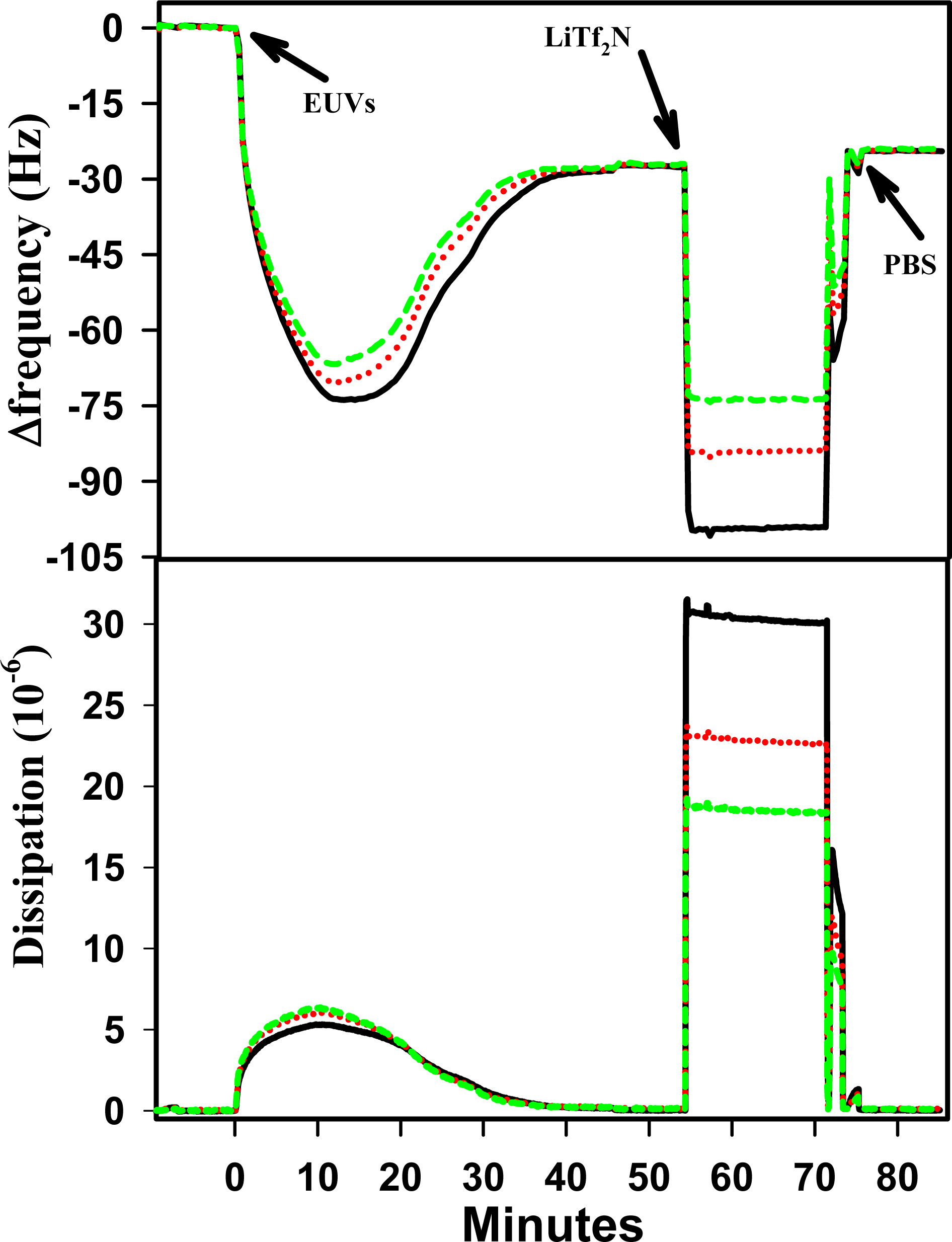
2.3 Result of BMP-Tf2N Interaction with SPB
2.4 Conclusions
3. Experimental Section
3.1. Extruded unilamellar vesicles preparation
3.2. Quartz crystal microbalance with dissipation monitoring
3.3 Atomic Force Microscopy
Acknowledgments
- †Product names are necessary to report factually on available data; however, the USDA neither guarantees nor warrants the standard of the product. In addition, the use of the name by the USDA implies no approval of the product to the exclusion of others that may be suitable.
References and Notes
- Hudson, EP; Eppler, RK; Clark, DS. Biocatalysis in semi-aqueous and nearly anhydrous conditions. Curr Opin Biotechnol 2005, 16, 637–643. [Google Scholar]
- Sardessai, YN; Bhosle, S. Industrial potential of organic solvent tolerant bacteria. Biotechnol Prog 2004, 20, 655–660. [Google Scholar]
- Wang, P; Dai, S; Waezsada, SD; Tsao, AY; Davison, BH. Enzyme stabilization by covalent binding in nanoporous sol-gel glass for nonaqueous biocatalysis. Biotechnol Bioeng 2001, 74, 249–255. [Google Scholar]
- Perez, C; Falero, A; Duc, HL; Balcinde, Y; Hung, BR. A very efficient bioconversion of soybean phytosterols mixtures to androstanes by mycobacteria. J Ind Microbiol Biotechnol 2006, 33, 719–723. [Google Scholar]
- Karboune, S; Safari, M; Lue, B-M; Yeboah, FK; Kermasha, S. Lipase-catalyzed biosynthesis of cinnamoylated lipids in a selected organic solvent medium. J Biotechnol 2005, 119, 281–290. [Google Scholar]
- Noureddini, H; Gao, X; Philkana, RS. Immobilized Pseudomonas cepacia lipase for biodiesel fuel production from soybean oil. Bioresourc Technol 2005, 96, 769–777. [Google Scholar]
- Quinn, BM; Ding, Z; Moulton, R; Bard, AJ. Novel Electrochemical Studies of Ionic Liquids. Langmuir 2002, 18, 1734–1742. [Google Scholar]
- Zhao, H; Malhotra, SV. Applications of ionic liquids in organic synthesis. Aldrichim Acta 2002, 35, 75–83. [Google Scholar]
- Xuebing, X. Engineering of enzymatic reactions and reactors for lipid modification and synthesis. Eur J Lipid Sci Technol 2003, 105, 289–304. [Google Scholar]
- Wu, Y; Hu, S. Biosensors based on direct electron transfer in redox proteins. Microchim Acta 2007, 159, 1–17. [Google Scholar]
- Pfruender, H; Jones, R; Weuster-Botz, D. Water immiscible ionic liquids as solvents for whole cell biocatalysis. J Biotechnol BioPerspectives 2006, 124, 182–190. [Google Scholar]
- Reetz, MT; Wiesenhofer, W; Francio, G; Leitner, W. Biocatalysis in ionic liquids: batchwise and continuous flow processes using supercritical carbon dioxide as the mobile phase. Chem Commun (Camb) 2002, 992–993. [Google Scholar]
- Laszlo, JA; Compton, DL. Alpha-chymotrypsin catalysis in imidazolium-based ionic liquids. Biotechnol Bioeng 2001, 75, 181–186. [Google Scholar]
- van Rantwijk, F. Lau, R.M.; Sheldon, R.A. Biocatalytic transformations in ionic liquids. Trends Biotechnol 2003, 21, 131–138. [Google Scholar]
- Yang, Z; Pan, W. Ionic liquids: Green solvents for nonaqueous biocatalysis. Enzyme Microb Technol 2005, 37, 19–28. [Google Scholar]
- Kragl, U; Eckstein, M; Kaftzik, N. Enzyme catalysis in ionic liquids. Curr Opin Biotechnol 2002, 13, 565–571. [Google Scholar]
- Laszlo, JA; Compton, DL. Comparison of peroxidase activities of hemin, cytochrome c and microperoxidase-11 in molecular solvents and imidazolium-based ionic liquids. J Mol Catal B Enzym 2002, 18, 109–120. [Google Scholar]
- Evans, KO. Room-temperature ionic liquid cations act as short-chain surfactants and disintegrate a phospholipid bilayer. Colloids Surf A 2005, 274, 11–17. [Google Scholar]
- Richter, RP; Berat, R; Brisson, AR. Formation of Solid-Supported Lipid Bilayers: An Integrated View. Langmuir 2006, 22, 3497–3505. [Google Scholar]
- Seantier, B; Breffa, C; Felix, O; Decher, G. In situ investigations of the formation of mixed supported lipid bilayers close to the phase transition temperature. Nano Lett 2004, 4, 5–10. [Google Scholar]
- Seantier, B; Breffa, C; Felix, O; Decher, G. Dissipation-Enhanced Quartz Crystal Microbalance Studies on the Experimental Parameters Controlling the Formation of Supported Lipid Bilayers. J Phys Chem B 2005, 109, 21755–21765. [Google Scholar]
- Boudard, S; Seantier, B; Breffa, C; Decher, G; Felix, O. Controlling the pathway of formation of supported lipid bilayers of DMPC by varying the sodium chloride concentration. Thin Solid Films 2006, 495, 246–251. [Google Scholar]
- Richter, R; Mukhopadhyay, A; Brisson, A. Pathways of Lipid Vesicle Deposition on Solid Surfaces: A Combined QCM-D and AFM Study. Biophys J 2003, 85, 3035–3047. [Google Scholar]
- Keller, CA; Kasemo, B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophy J 1998, 75, 1397–1402. [Google Scholar]
- Sauerbrey, G. The use of quartz oscillators for weighing thin layers and for microweighing. Z Phys 1959, 155, 206–222. [Google Scholar]
- Reimhult, E; Hook, F; Kasemo, B. Vesicle Adsorption on SiO2 and TiO2: Dependence on Vesicle Size. J Chem Phys 2002, 117, 7401–7404. [Google Scholar]
- Hook, F; Kasemo, B; Nylander, T; Fant, C; Sott, K; Elwing, H. Variations in coupled water, viscoelastic properties, and film thickness of a Mefp-1 protein film during adsorption and cross-linking: a quartz crystal microbalance with dissipation monitoring, ellipsometry, and surface plasmon resonance study. Anal Chem 2001, 73, 5796–5804. [Google Scholar]
- Daikhin, L; Urbakh, M. Effect of Surface Film Structure on the Quartz Crystal Microbalance Response in Liquids. Langmuir 1996, 12, 6354–6360. [Google Scholar]
- Leonenko, ZV; Carnini, A; Cramb, DT. Supported planar bilayer formation by vesicle fusion: the interaction of phospholipid vesicles with surfaces and the effect of gramicidin on bilayer properties using atomic force microscopy. Biochim Biophys Acta Biomemb 2000, 1509, 131–147. [Google Scholar]
- Singh, T; Kumar, A. Aggregation Behavior of Ionic Liquids in Aqueous Solutions: Effect of Alkyl Chain Length, Cations, and Anions. J Phys Chem B 2007, 111, 7843–7851. [Google Scholar]
- Bowers, J; Butts, CP; Martin, PJ; Vergara-Gutierrez, MC. Aggregation Behavior of Aqueous Solutions of Ionic Liquids. Langmuir 2004, 20, 2191–2198. [Google Scholar]
- Leroueil, PR; Berry, SA; Duthie, K; Han, G; Rotello, VM; McNerny, DQ; Baker, JR; Orr, BG; BanaszakHoll, MM. Wide Varieties of Cationic Nanoparticles Induce Defects in Supported Lipid Bilayers. Nano Lett 2008, 8, 420–424. [Google Scholar]
- Mecke, A; Majoros, IJ; Patri, AK; Baker, JR; BanaszakHoll, MM; Orr, BG. Lipid Bilayer Disruption by Polycationic Polymers: The Roles of Size and Chemical Functional Group. Langmuir 2005, 21, 10348–10354. [Google Scholar]
- Hong, S; Bielinska, AU; Mecke, A; Keszler, B; Beals, JL; Shi, X; Balogh, L; Orr, BG; Baker, JR; BanaszakHoll, MM. Interaction of Poly(amidoamine) Dendrimers with Supported Lipid Bilayers and Cells: Hole Formation and the Relation to Transport. Bioconjugate Chem 2004, 15, 774–782. [Google Scholar]
- Patel, AR; Frank, CW. Quantitative Analysis of Tethered Vesicle Assemblies by Quartz Crystal Microbalance with Dissipation Monitoring: Binding Dynamics and Bound Water Content. Langmuir 2006, 22, 7587–7599. [Google Scholar]
- Rodahl, M; Kasemo, B. A simple setup to simultaneously measure the resonant frequency and the absolute dissipation factor of a quartz crystal microbalance. Rev Sci Instrum 1996, 67, 3238–3241. [Google Scholar]
- Krozer, A; Rodahl, M. X-ray photoemission spectroscopy study of UV/ozone oxidation of Au under ultrahigh vacuum conditions. J Vac Sci Technol A 1997, 15, 1704–1709. [Google Scholar]
- Harewood, K; Wolff, JSI. A rapid electrophoretic procedure for the detection of SDS-released oncorna-viral RNA using polyacrylamide-agarose gels. Anal Biochem 1973, 55, 573–581. [Google Scholar]
- Penfold, J; Staples, E; Tucker, I; Thomas, RK. Adsorption of Mixed Anionic and Nonionic Surfactants at the Hydrophilic Silicon Surface. Langmuir 2002, 18, 5755–5760. [Google Scholar]
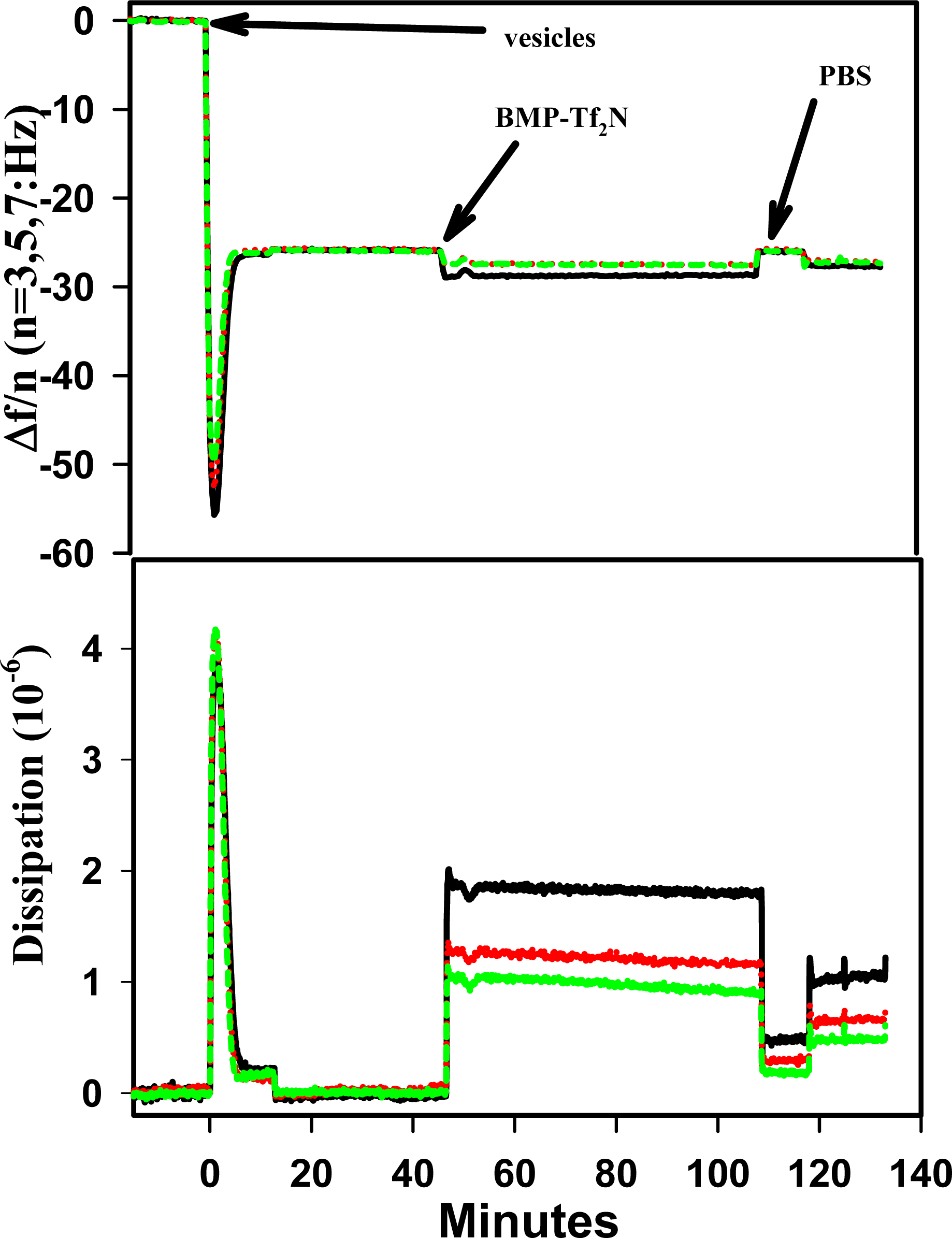
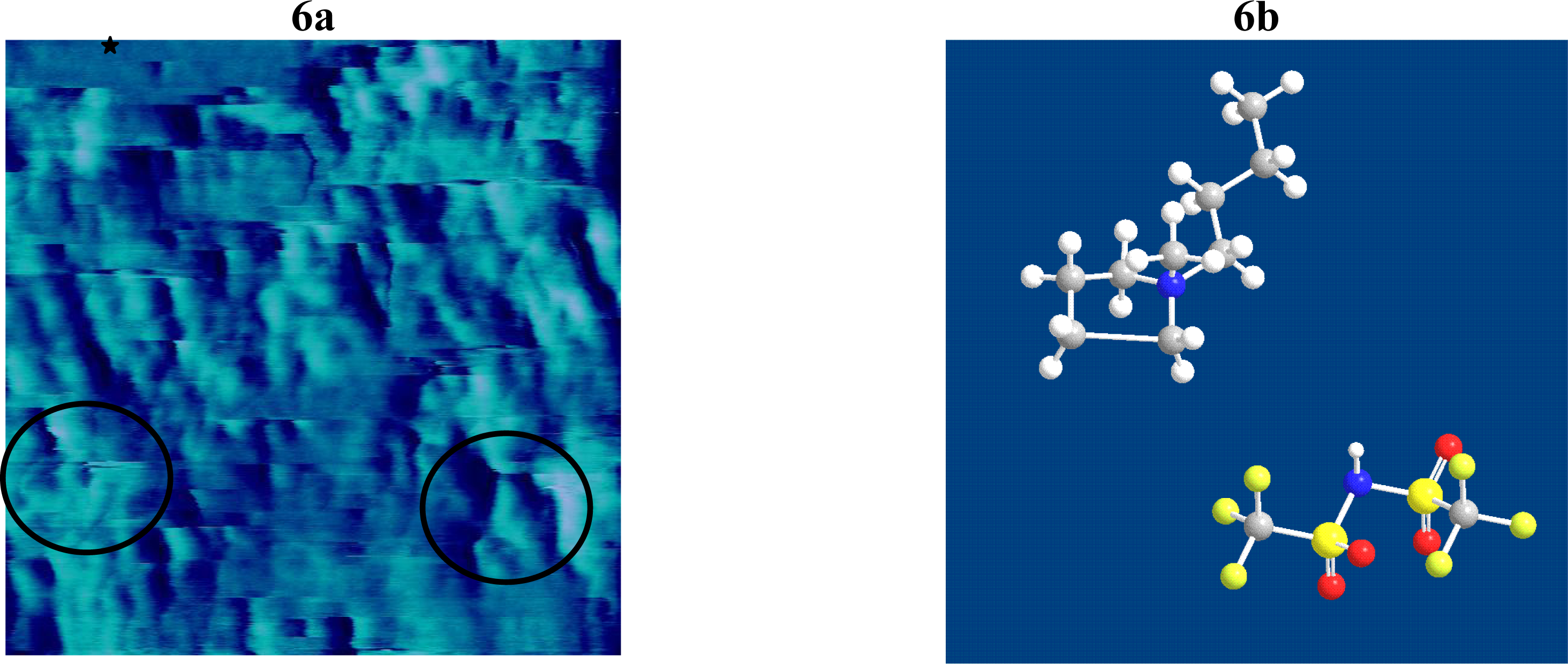
| RT-IL Component | Δ f3 (Hz) a, b | Δ D3 (10-6) b | Mass (ng/cm2) c | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| OMIM+ | –29.0 ± 3.2 | –24.2 ± 5.5 | 0.61 ± 1.2 | 1.1 ± 2.1 | 522 ± 58 | 437 ± 97 |
| Tf2N- | –30.0 ± 2.6 | –25.0 ± 2.4 | 0.36 ± 0.47 | 0.026 ± 0.005 | 542 ± 32 | 452 ± 20 |
| BMP-Tf2N | –26.0 ± 0.1 | –28.2 ± 0.8 | 0.03 ± 0.08 | 1.1 ± 0.1 | 460 ± 3 | 500 ± 14 |
Share and Cite
Evans, K.O. Supported Phospholipid Bilayer Interaction with Components Found in Typical Room-Temperature Ionic Liquids – a QCM-D and AFM Study. Int. J. Mol. Sci. 2008, 9, 498-511. https://doi.org/10.3390/ijms9040498
Evans KO. Supported Phospholipid Bilayer Interaction with Components Found in Typical Room-Temperature Ionic Liquids – a QCM-D and AFM Study. International Journal of Molecular Sciences. 2008; 9(4):498-511. https://doi.org/10.3390/ijms9040498
Chicago/Turabian StyleEvans, Kervin O. 2008. "Supported Phospholipid Bilayer Interaction with Components Found in Typical Room-Temperature Ionic Liquids – a QCM-D and AFM Study" International Journal of Molecular Sciences 9, no. 4: 498-511. https://doi.org/10.3390/ijms9040498
APA StyleEvans, K. O. (2008). Supported Phospholipid Bilayer Interaction with Components Found in Typical Room-Temperature Ionic Liquids – a QCM-D and AFM Study. International Journal of Molecular Sciences, 9(4), 498-511. https://doi.org/10.3390/ijms9040498




