N, N-Dimethyl-4-Aminopyridine- and Aluminum Isopropoxide-Catalysed Ring-Opening Polymerizations of β-Butyrolactone for the Antimicrobial Oligohydroxybutyrate
Abstract
1. Introduction
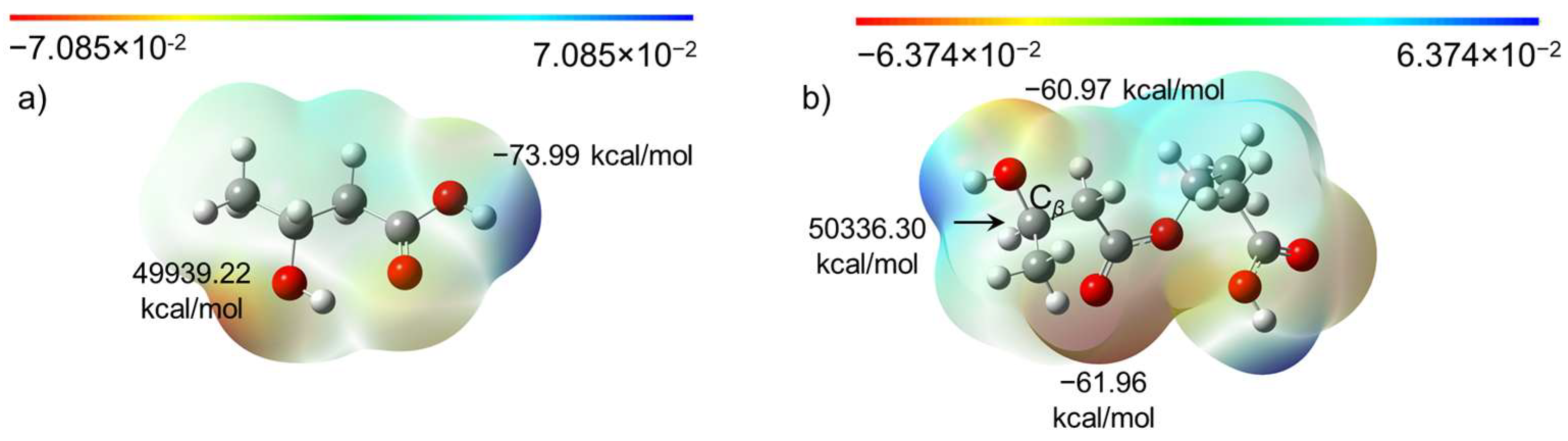
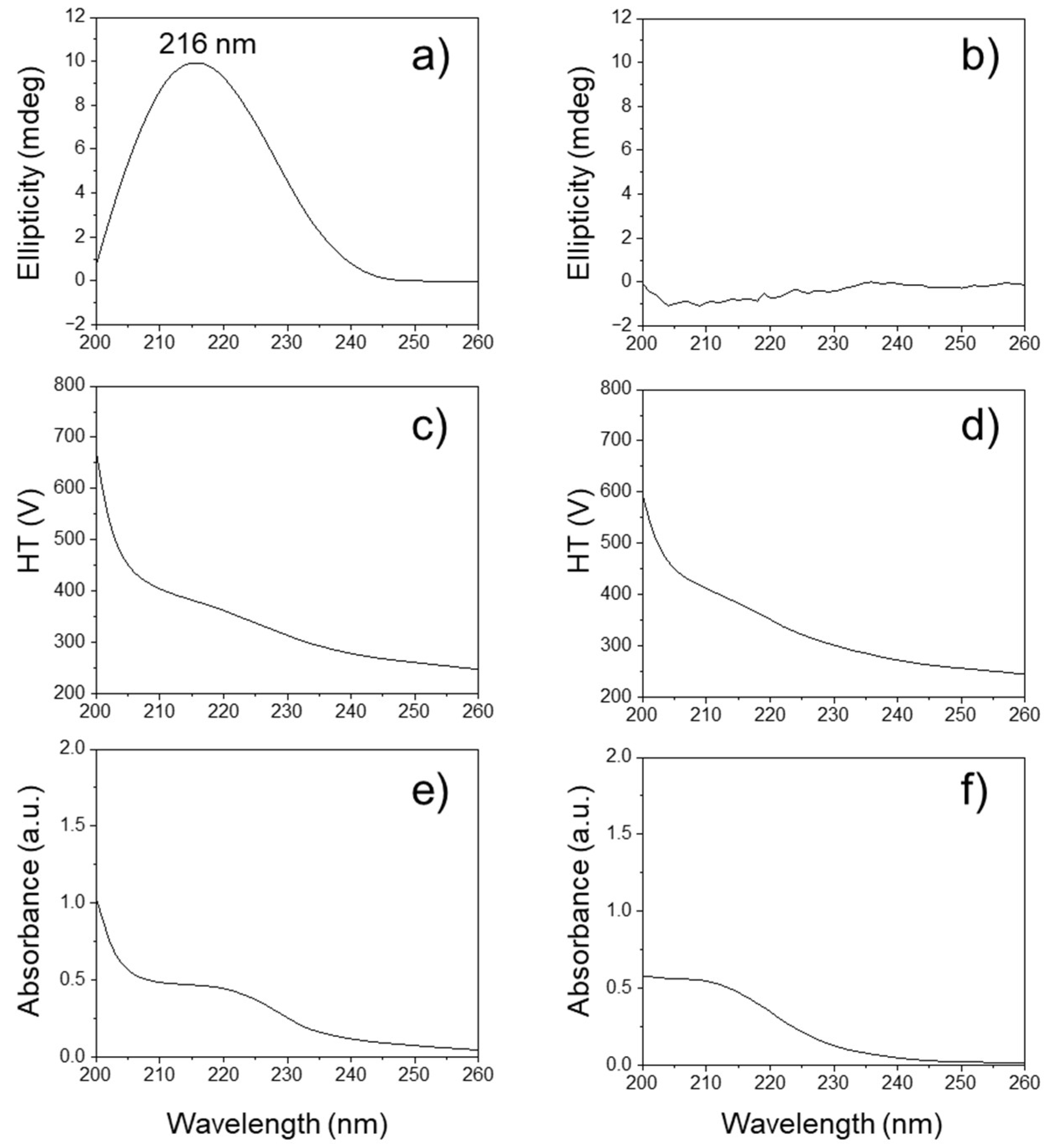
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Characterization
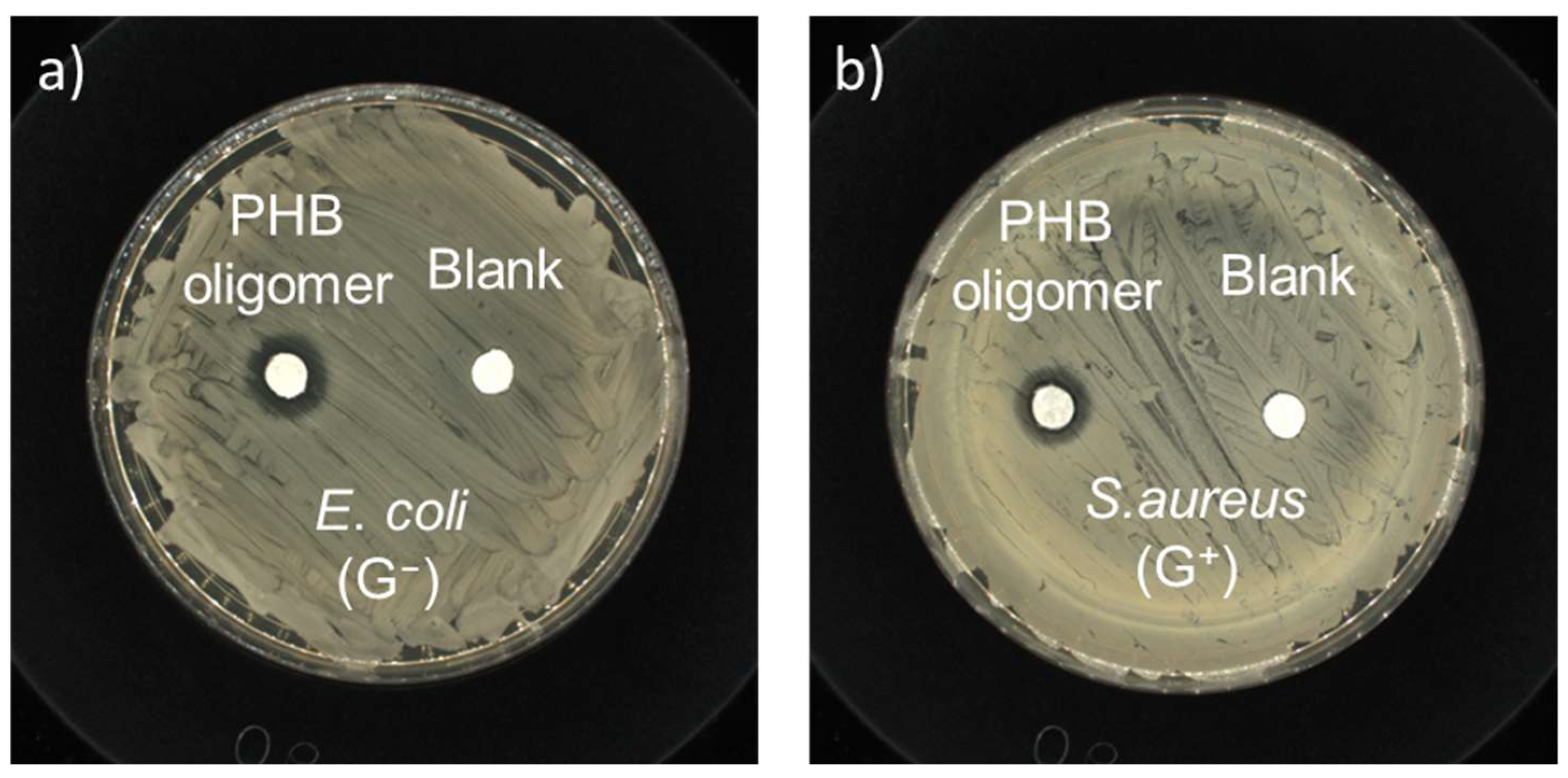
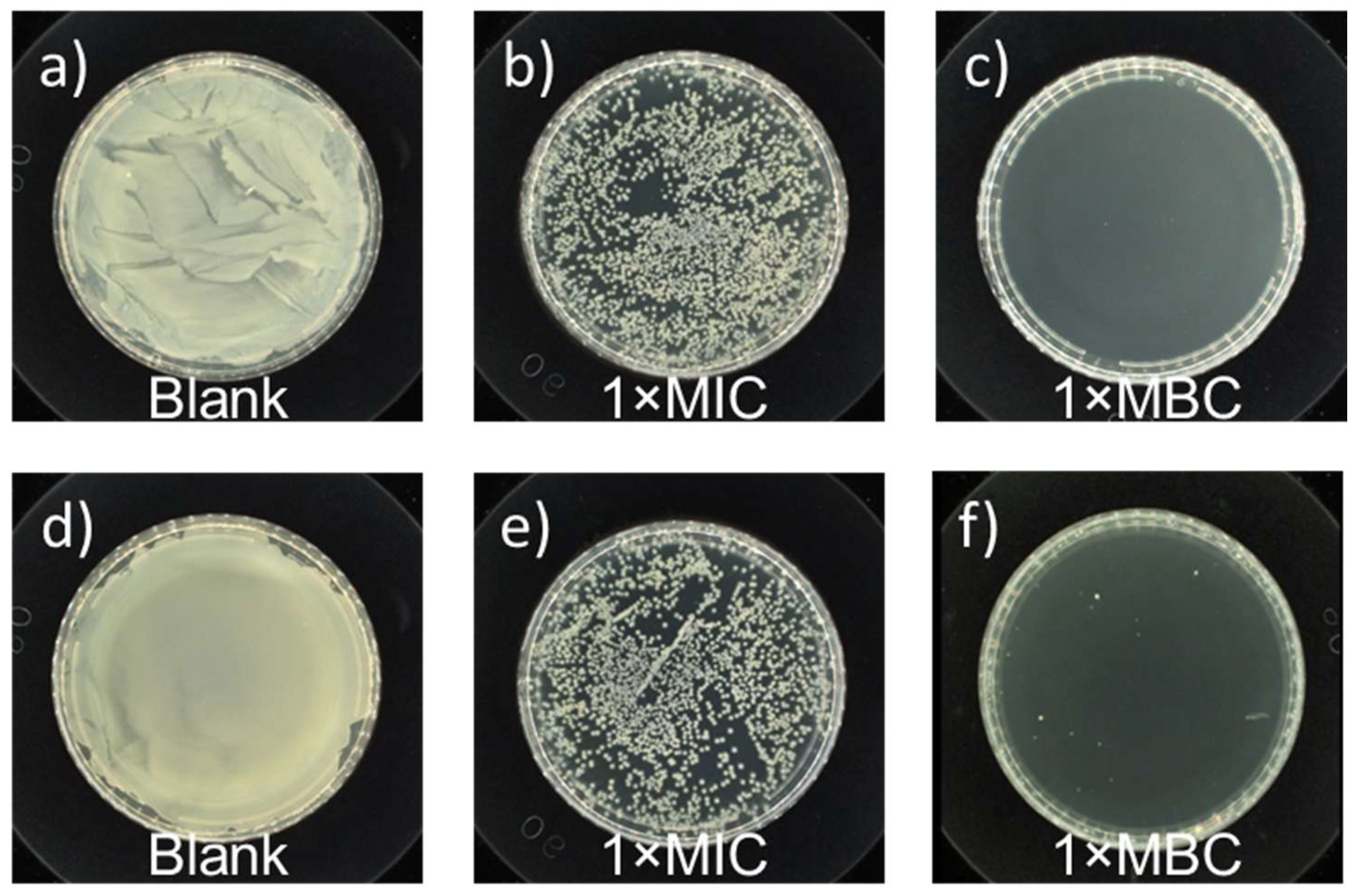
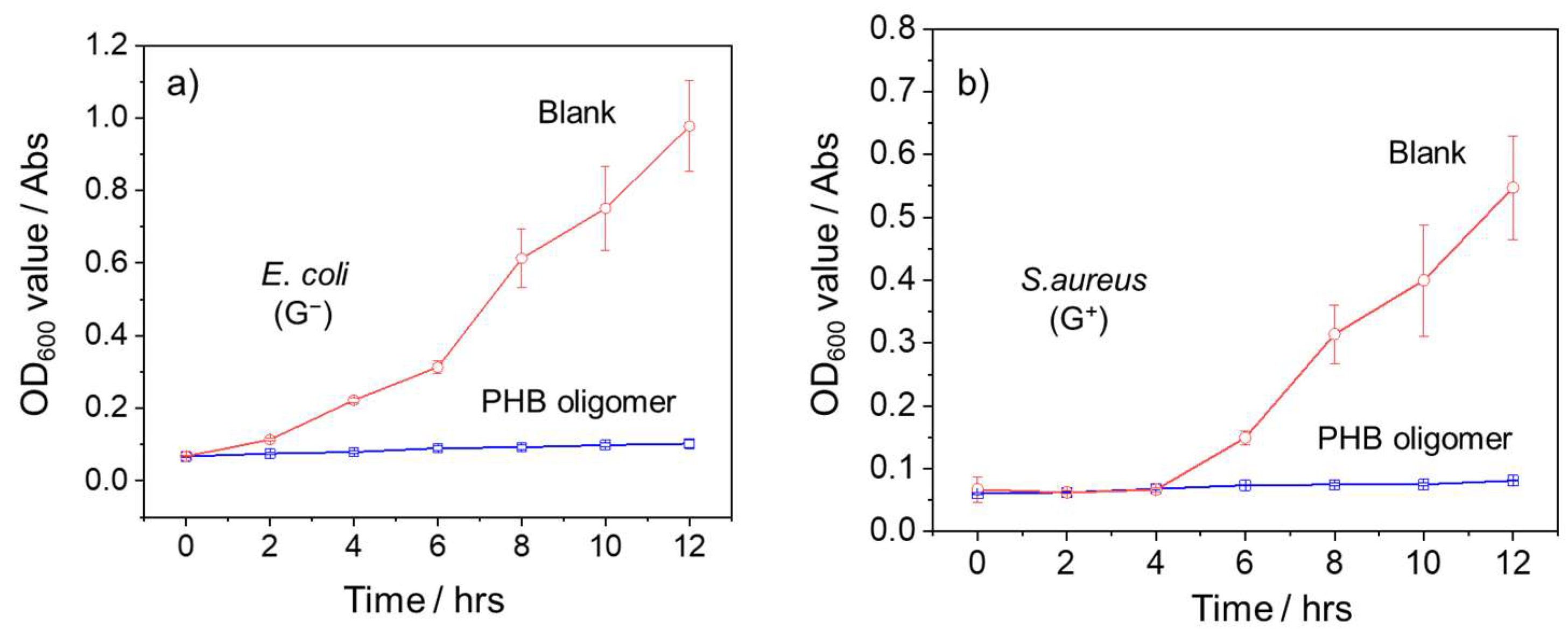
3.3. Antimicrobial Activity Evaluation
4. Conclusions
- DMAP and Al(OiPr)3 were employed to improve the ROP reaction pertaining to PHB. DMAP displayed more advantages over pyridine in terms of catalytic efficiency and polymer degree, and it could also initiate the polymerization of BL alone.
- The FT-IR, 1H and 13C NMR, and H-ESI MS/MS characterization associated with chemistry-computing calculations revealed the loss of terminal hydroxyl groups of the PHB oligomers.
- The as-prepared PHB oligomers showed rapid and broad-spectrum antimicrobial effects against a wide range of microbes, ranging from viruses, including influenza A virus (H1N1 and H3N2) and SARS-CoV-2 (COVID-19), to bacteria, including E. coli and S. aureus.
- Furthermore, leveraging the side products of ROP with crotonate end-capping groups endowed PHB with more opportunities via end-chain functionalization in the post-copolymerization process.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohanty, A.K.; Wu, F.; Mincheva, R.; Hakkarainen, M.; Raquez, J.-M.; Mielewski, D.F.; Narayan, R.; Netravali, A.N.; Misra, M. Sustainable Polymers. Nat. Rev. Methods Primers 2022, 2, 46. [Google Scholar] [CrossRef]
- Grillo, A.; Rusconi, Y.; D’Alterio, M.C.; De Rosa, C.; Talarico, G.; Poater, A. Ring Opening Polymerization of Six- and Eight-Membered Racemic Cyclic Esters for Biodegradable Materials. Int. J. Mol. Sci. 2024, 25, 1647. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Afanaseva, A.; Vinogradov, A.; Ivchenko, P. Unsaturated Macrolactones from Renewable Feedstocks: Synthesis, Ring-Opening Polymerization and Application Prospects. Int. J. Mol. Sci. 2025, 26, 5039. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, Z.; Luo, H.; Tao, X. Evaluating and Modeling the Degradation of PLA/PHB Fabrics in Marine Water. Polymers 2023, 15, 82. [Google Scholar] [CrossRef]
- Bao, Q.; Wong, W.; Liu, S.; Tao, X. Accelerated Degradation of Poly(Lactide Acid)/Poly(Hydroxybutyrate) (PLA/PHB) Yarns/Fabrics by UV and O2 Exposure in South China Seawater. Polymers 2022, 14, 1216. [Google Scholar] [CrossRef] [PubMed]
- Kopf, S.; Åkesson, D.; Hakkarainen, M.; Skrifvars, M. Effect of Hydroxyapatite Particle Morphology on As-Spun Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Hydroxyapatite Composite Fibers. Results Mater. 2023, 20, 100465. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Z.; Shi, C.; Scoti, M.; Barange, D.K.; Gowda, R.R.; Chen, E.Y.-X. Chemically Circular, Mechanically Tough, and Melt-Processable Polyhydroxyalkanoates. Science 2023, 380, 64–69. [Google Scholar] [CrossRef]
- Huang, X.; Tao, X.; Zhang, Z.; Chen, P. Properties and Performances of Fabrics Made from Bio-Based and Degradable Polylactide Acid/Poly (Hydroxybutyrate-Co-Hydroxyvalerate) (PLA/PHBV) Filament Yarns. Text. Res. J. 2017, 87, 2464–2474. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Z.; Li, J.; Yang, X.; Fei, B.; Leung, P.H.M.; Tao, X. A New Antimicrobial Agent: Poly (3-Hydroxybutyric Acid) Oligomer. Macromol. Biosci. 2019, 19, e1800432. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Ma, L.; Yang, X.; Fei, B.; Leung, P.H.M.; Tao, X. Mechanistic Study of Synergistic Antimicrobial Effects between Poly (3-Hydroxybutyrate) Oligomer and Polyethylene Glycol. Polymers 2020, 12, 2735. [Google Scholar] [CrossRef]
- Adamus, G.; Domiński, A.; Kowalczuk, M.; Kurcok, P.; Radecka, I. From Anionic Ring-Opening Polymerization of β-Butyrolactone to Biodegradable Poly(Hydroxyalkanoate)s: Our Contributions in This Field. Polymers 2021, 13, 4365. [Google Scholar] [CrossRef]
- Yan, X.; Liu, X.-Y.; Zhang, D.; Zhang, Y.-D.; Li, Z.-H.; Liu, X.; Wu, F.; Chen, G.-Q. Construction of a Sustainable 3-Hydroxybutyrate-Producing Probiotic Escherichia Coli for Treatment of Colitis. Cell. Mol. Immunol. 2021, 18, 2344–2357. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, Y.; Huang, W.; Zhang, L.; Wu, F.; Ye, J.; Chen, G.-Q. Rational Flux-Tuning of Halomonas Bluephagenesis for Co-Production of Bioplastic PHB and Ectoine. Nat. Commun. 2020, 11, 3313. [Google Scholar] [CrossRef]
- Li, M.; Ma, Y.; Zhang, X.; Zhang, L.; Chen, X.; Ye, J.-W.; Chen, G.-Q. Tailor-Made Polyhydroxyalkanoates by Reconstructing Pseudomonas Entomophila. Adv. Mater. 2021, 33, 2102766. [Google Scholar] [CrossRef]
- Quinn, E.C.; Parker, C.R.; Guillaume, S.M.; Chen, E.Y.-X. Regulating the Stereomicrostructure, Circularity and Functionality of Synthetic PHAs. Polym. Chem. 2025, 16, 512–520. [Google Scholar] [CrossRef]
- Carpentier, J.-F. Discrete Metal Catalysts for Stereoselective Ring-Opening Polymerization of Chiral Racemic β-Lactones. Macromol. Rapid Commun. 2010, 31, 1696–1705. [Google Scholar] [CrossRef]
- Caputo, M.R.; Tang, X.; Westlie, A.H.; Sardon, H.; Chen, E.Y.-X.; Müller, A.J. Effect of Chain Stereoconfiguration on Poly(3-Hydroxybutyrate) Crystallization Kinetics. Biomacromolecules 2022, 23, 3847–3859. [Google Scholar] [CrossRef] [PubMed]
- Vagin, S.; Winnacker, M.; Kronast, A.; Altenbuchner, P.T.; Deglmann, P.; Sinkel, C.; Loos, R.; Rieger, B. New Insights into the Ring-Opening Polymerization of β-Butyrolactone Catalyzed by Chromium(III) Salphen Complexes. ChemCatChem 2015, 7, 3963–3971. [Google Scholar] [CrossRef]
- Reichardt, R.; Vagin, S.; Reithmeier, R.; Ott, A.K.; Rieger, B. Factors Influencing the Ring-Opening Polymerization of Racemic β-Butyrolactone Using CrIII(Salphen). Macromolecules 2010, 43, 9311–9317. [Google Scholar] [CrossRef]
- Silvino, A.C.; da Silva, J.C. Preparation of Blends of Oligo([R,S]-3-Hydroxybutyrate) and Poly([R]-3-Hydroxybutyrate): Thermal Properties and Molecular Dynamic Studies. Polym. Test. 2015, 42, 144–150. [Google Scholar] [CrossRef]
- Hiki, S.; Miyamoto, M.; Kimura, Y. Synthesis and Characterization of Hydroxy-Terminated [RS]-Poly(3-Hydroxybutyrate) and Its Utilization to Block Copolymerization with l-Lactide to Obtain a Biodegradable Thermoplastic Elastomer. Polymer 2000, 41, 7369–7379. [Google Scholar] [CrossRef]
- Barouti, G.; Jaffredo, C.G.; Guillaume, S.M. Advances in Drug Delivery Systems Based on Synthetic Poly(Hydroxybutyrate) (Co)Polymers. Prog. Polym. Sci. 2017, 73, 1–31. [Google Scholar] [CrossRef]
- Altenbuchner, P.T.; Kronast, A.; Kissling, S.; Vagin, S.I.; Herdtweck, E.; Pöthig, A.; Deglmann, P.; Loos, R.; Rieger, B. Mechanistic Investigations of the Stereoselective Rare Earth Metal-Mediated Ring-Opening Polymerization of β-Butyrolactone. Chem. Eur. J. 2015, 21, 13609–13617. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.; Ebrahimi, T.; Hatzikiriakos, S.G.; Mehrkhodavandi, P. Star-Shaped PHB–PLA Block Copolymers: Immortal Polymerization with Dinuclear Indium Catalysts. Dalton Trans. 2015, 44, 14248–14254. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yu, I.; Mehrkhodavandi, P. Highly Controlled Immortal Polymerization of β-Butyrolactone by a Dinuclear Indium Catalyst. Chem. Commun. 2012, 48, 6806–6808. [Google Scholar] [CrossRef]
- Jiang, J.; Choi, J.; Yoon, S. Living Ring-Opening Polymerization of β-Butyrolactone Initiated by Mononuclear Zirconium Compounds Containing Sterically Hindered N,O-Chelate and Anionic Dimethylamide Ligands. RSC Adv. 2023, 13, 10379–10383. [Google Scholar] [CrossRef] [PubMed]
- Hornberger, L.-S.; Weingarten, P.; Lange, P.L.; Schleid, T.; Adams, F. Connecting 16- with 4-Membered Rings: Ring-Opening Polymerization of Bio-Based ω-Pentadecalactone with Amino-Alkoxy-Bis(Phenolate) Yttrium Initiators and Copolymerization with β-Butyrolactone. Eur. Polym. J. 2023, 199, 112449. [Google Scholar] [CrossRef]
- Omar, R.; Shaik, M.; Griggs, C.; Jensen, J.D.; Boyd, R.; Oncel, N.; Webster, D.C.; Du, G. Star-Shaped Poly(Hydroxybutyrate)s from Bio-Based Polyol Cores via Zinc Catalyzed Ring-Opening Polymerization of β-Butyrolactone. Eur. Polym. J. 2021, 160, 110756. [Google Scholar] [CrossRef]
- Billingham, N.C.; Proctor, M.G.; Smith, J.D. Polymerization and Copolymerization of β-Butyrolactone by Aluminium Compounds. J. Organomet. Chem. 1988, 341, 83–93. [Google Scholar] [CrossRef]
- Guillaume, S.M.; Annunziata, L.; del Rosal, I.; Iftner, C.; Maron, L.; Roesky, P.W.; Schmid, M. Ring-Opening Polymerization of Racemic β-Butyrolactone Promoted by Rare Earth Trisborohydride Complexes towards a PHB-Diol: An Experimental and DFT Study. Polym. Chem. 2013, 4, 3077–3087. [Google Scholar] [CrossRef]
- Bruckmoser, J.; Pongratz, S.; Stieglitz, L.; Rieger, B. Highly Isoselective Ring-Opening Polymerization of Rac-β-Butyrolactone: Access to Synthetic Poly(3-Hydroxybutyrate) with Polyolefin-like Material Properties. J. Am. Chem. Soc. 2023, 145, 11494–11498. [Google Scholar] [CrossRef] [PubMed]
- Barczyńska-Felusiak, R.; Pastusiak, M.; Rychter, P.; Kaczmarczyk, B.; Sobota, M.; Wanic, A.; Kaps, A.; Jaworska-Kik, M.; Orchel, A.; Dobrzyński, P. Synthesis of the Bacteriostatic Poly(l-Lactide) by Using Zinc (II)[(Acac)(L)H2O] (L = Aminoacid-Based Chelate Ligands) as an Effective ROP Initiator. Int. J. Mol. Sci. 2021, 22, 6950. [Google Scholar] [CrossRef] [PubMed]
- Kovács, E.; Balterer, B.; Anh Duc, N.; Szarka, G.; Owen, M.C.; Domján, A.; Iván, B. Ring-Opening Metathesis Polymerization and Related Olefin Metathesis Reactions in Benzotrifluoride as an Environmentally Advantageous Medium. Int. J. Mol. Sci. 2023, 24, 671. [Google Scholar] [CrossRef] [PubMed]
- Aida, T.; Inoue, S. Metalloporphyrins as Initiators for Living and Immortal Polymerizations. Acc. Chem. Res. 1996, 29, 39–48. [Google Scholar] [CrossRef]
- Young, M.S.; LaPointe, A.M.; MacMillan, S.N.; Coates, G.W. Highly Enantioselective Polymerization of β-Butyrolactone by a Bimetallic Magnesium Catalyst: An Interdependent Relationship Between Favored and Unfavored Enantiomers. J. Am. Chem. Soc. 2024, 146, 18032–18040. [Google Scholar] [CrossRef]
- Bero, M.; Kasperczyk, J.; Jedlinski, Z.J. Coordination Polymerization of Lactides, 1. Structure Determination of Obtained Polymers. Makromol. Chem. 1990, 191, 2287–2296. [Google Scholar] [CrossRef]
- Matsubara, K.; Eda, K.; Ikutake, Y.; Dan, M.; Tanizaki, N.; Koga, Y.; Yasuniwa, M. Aluminum Complex Initiated Copolymerization of Lactones and DL-Lactide to Form Crystalline Gradient Block Copolymers Containing Stereoblock Lactyl Chains. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2536–2544. [Google Scholar] [CrossRef]
- Palenzuela, M.; Mula, E.; Blanco, C.; Sessini, V.; Shakaroun, R.M.; Li, H.; Guillaume, S.M.; Mosquera, M.E.G. Copolymerization of β-Butyrolactones into Functionalized Polyhydroxyalkanoates Using Aluminum Catalysts: Influence of the Initiator in the Ring-Opening Polymerization Mechanism. Macromol. Rapid Commun. 2024, 45, e2400091. [Google Scholar] [CrossRef]
- Beament, J.; Mahon, M.F.; Buchard, A.; Jones, M.D. Aluminum Complexes of Monopyrrolidine Ligands for the Controlled Ring-Opening Polymerization of Lactide. Organometallics 2018, 37, 1719–1724. [Google Scholar] [CrossRef]
- Nederberg, F.; Connor, E.F.; Möller, M.; Glauser, T.; Hedrick, J.L. New Paradigms for Organic Catalysts: The First Organocatalytic Living Polymerization. Angew. Chem. Int. Ed. 2001, 40, 2712–2715. [Google Scholar] [CrossRef]
- Kamber, N.E.; Jeong, W.; Waymouth, R.M.; Pratt, R.C.; Lohmeijer, B.G.G.; Hedrick, J.L. Organocatalytic Ring-Opening Polymerization. Chem. Rev. 2007, 107, 5813–5840. [Google Scholar] [CrossRef] [PubMed]
- Jaipuri, F.A.; Bower, B.D.; Pohl, N.L. Protic Acid-Catalyzed Polymerization of β-Lactones for the Synthesis of Chiral Polyesters. Tetrahedron. Asymmetry 2003, 14, 3249–3252. [Google Scholar] [CrossRef]
- Kontogiannopoulos, K.N.; Barmpalexis, P. Closing Editorial: Advanced Polymeric Materials for Pharmaceutical Applications III. Polymers 2024, 16, 3004. [Google Scholar] [CrossRef]
- Clark, J.M.; Pilath, H.M.; Mittal, A.; Michener, W.E.; Robichaud, D.J.; Johnson, D.K. Direct Production of Propene from the Thermolysis of Poly(β-Hydroxybutyrate) (PHB). An Experimental and DFT Investigation. J. Phys. Chem. A 2016, 120, 332–345. [Google Scholar] [CrossRef]
- Nugroho, A.E.; Morita, H. Circular Dichroism Calculation for Natural Products. J. Nat. Med. 2014, 68, 1–10. [Google Scholar] [CrossRef]
- Rueping, M.; Dietrich, A.; Buschmann, V.; Fritz, M.G.; Sauer, M.; Seebach, D. On the Structure of Poly(3-Hydroxybutanoic Acid) in Solution and in Phospholipid Bilayers. Circular Dichroism and Fluorescence Spectroscopy with Oligo(3-Hydroxybutanoic Acid) Derivatives. Macromolecules 2001, 34, 7042–7048. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, Z.; Yu, B.; Sun, H.; Leung, P.H.; Tao, X. Synthesis of Polylactic Acid Oligomers for Broad-Spectrum Antimicrobials. Polymers 2022, 14, 4399. [Google Scholar] [CrossRef]
- Lowe, R.; Glen, R.C.; Mitchell, J.B.O. Predicting Phospholipidosis Using Machine Learning. Mol. Pharm. 2010, 7, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Frallicciardi, J.; Gabba, M.; Poolman, B. Determining Small-Molecule Permeation through Lipid Membranes. Nat. Protoc. 2022, 17, 2620–2646. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, T. A Comprehensive Electron Wavefunction Analysis Toolbox for Chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, D.; Qi, P. Synthesis and Characterization of Silver Nanoparticle and Graphene Oxide Nanosheet Composites as a Bactericidal Agent for Water Disinfection. J. Colloid Interface Sci. 2011, 360, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Muench, H.R. A Simple Method of Estimating 50 per Cent End Points. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Lei, C.; Yang, J.; Hu, J.; Sun, X. On the Calculation of TCID50 for Quantitation of Virus Infectivity. Virol. Sin. 2021, 36, 141–144. [Google Scholar] [CrossRef]
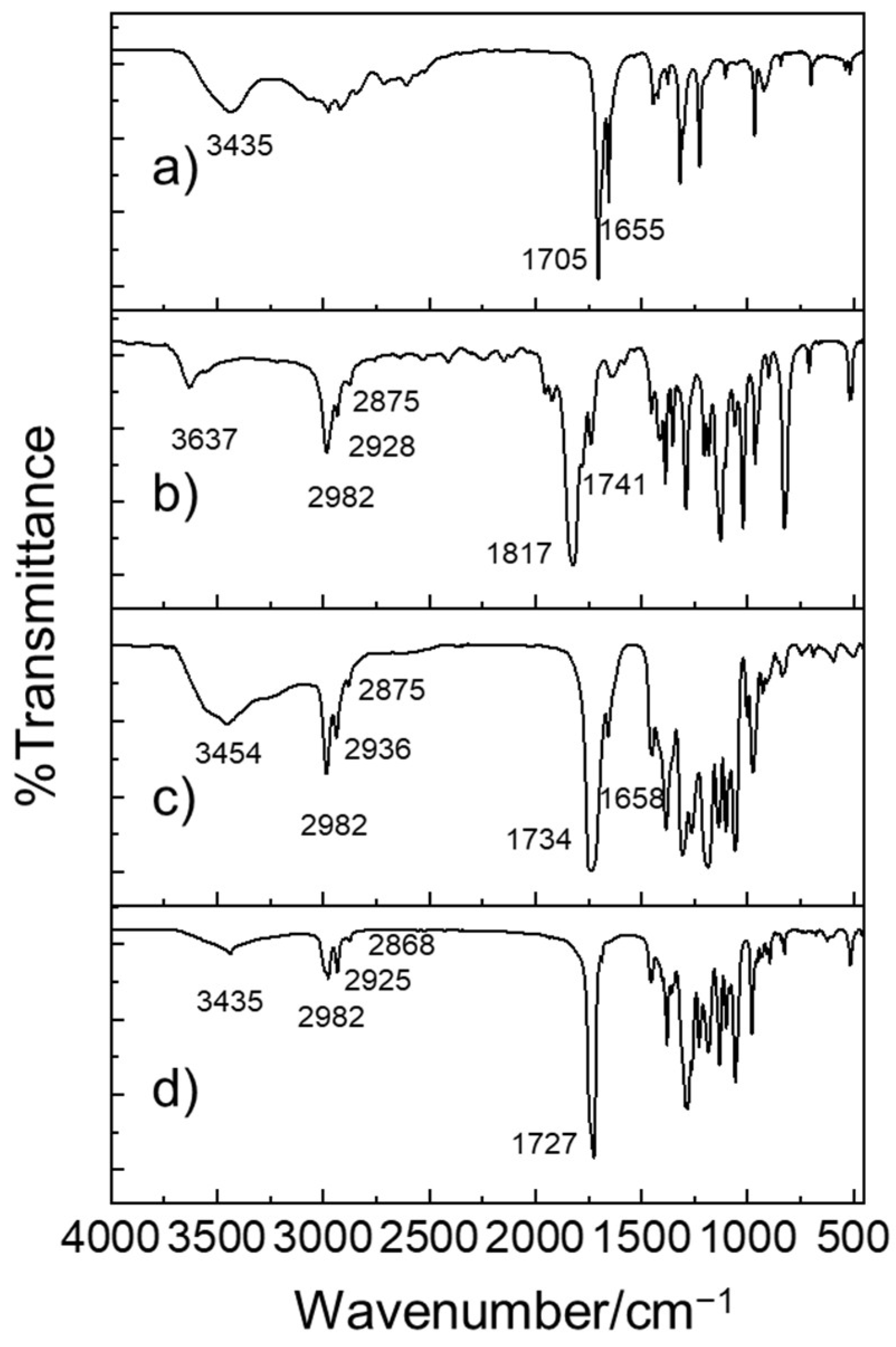
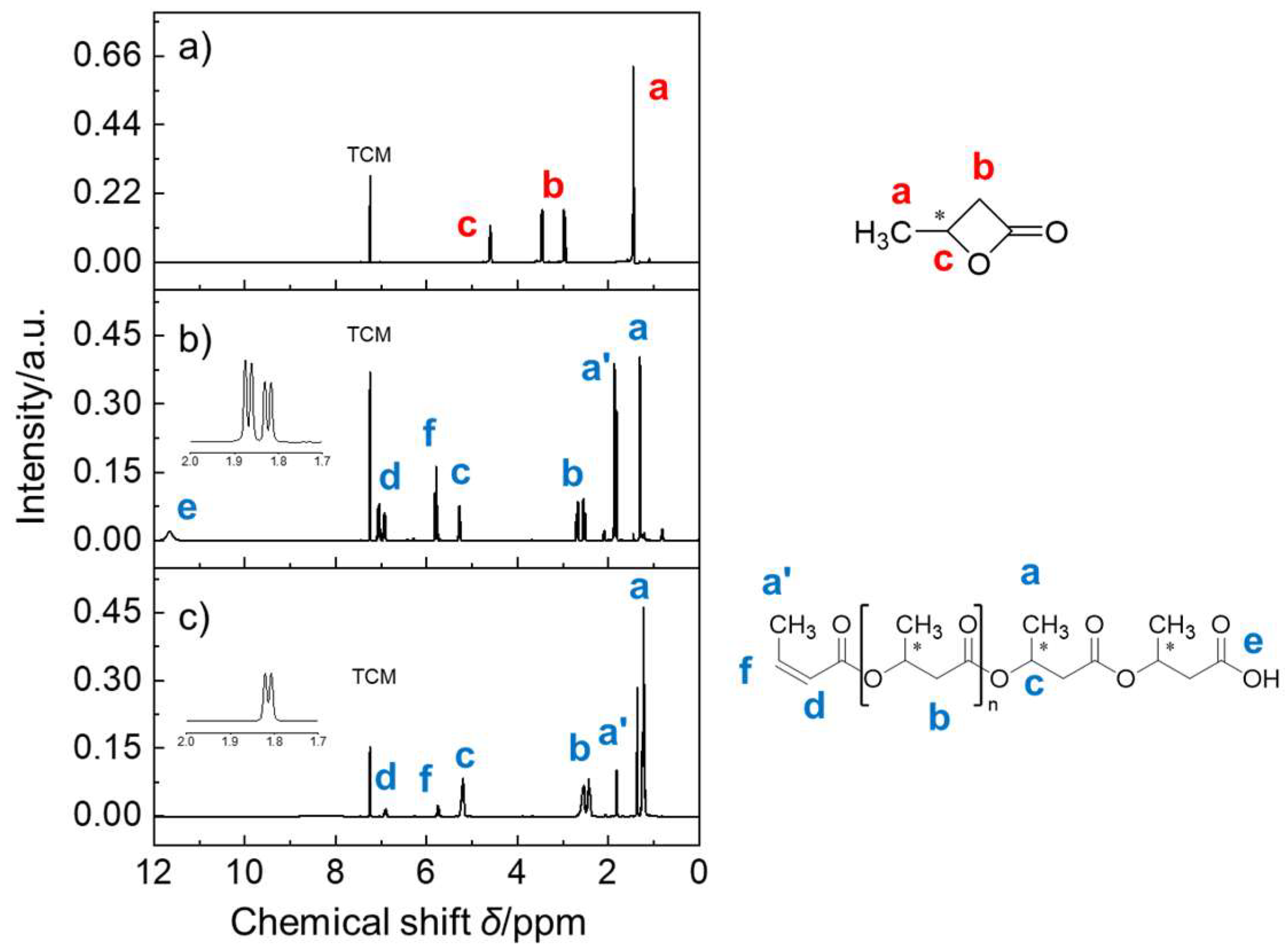
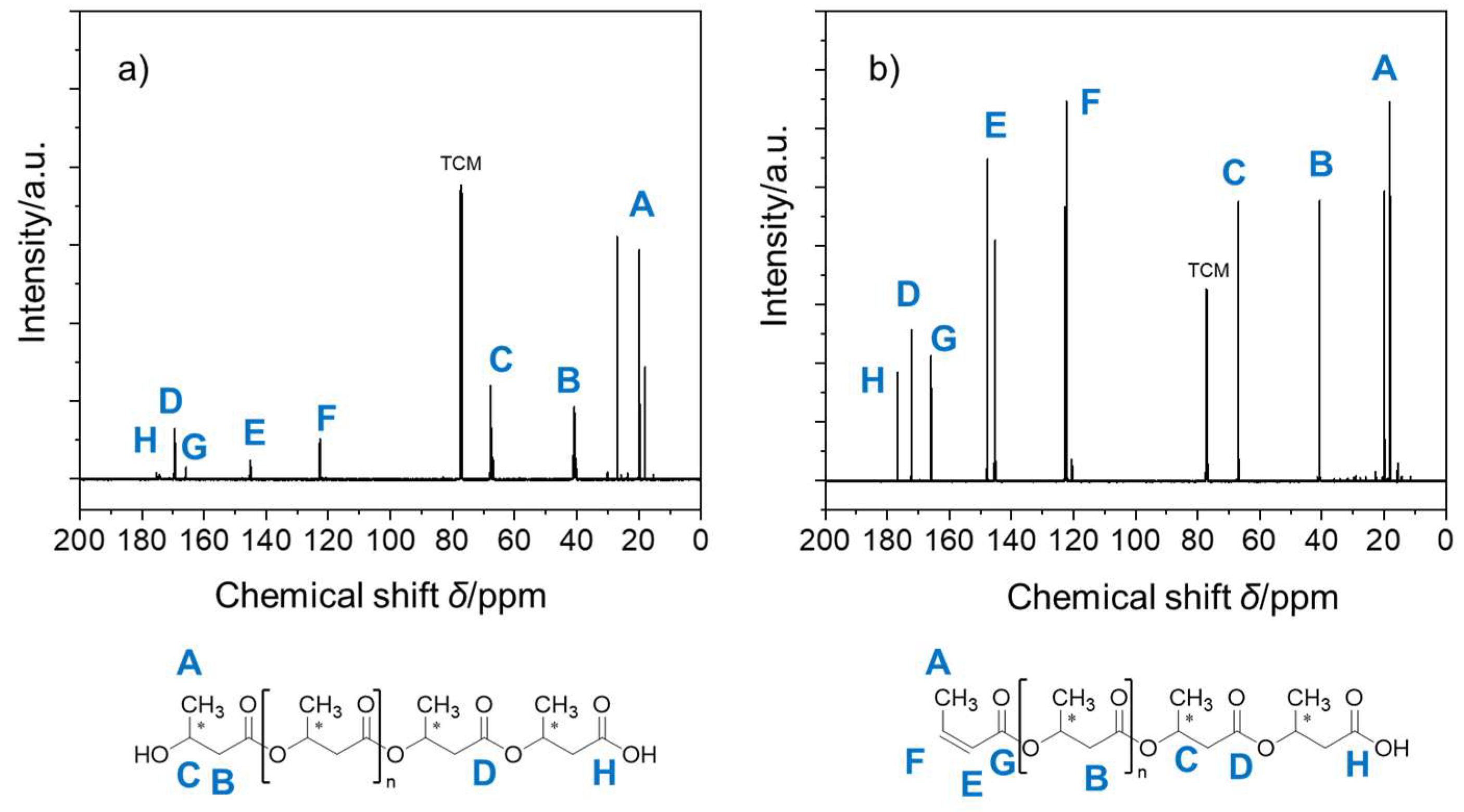
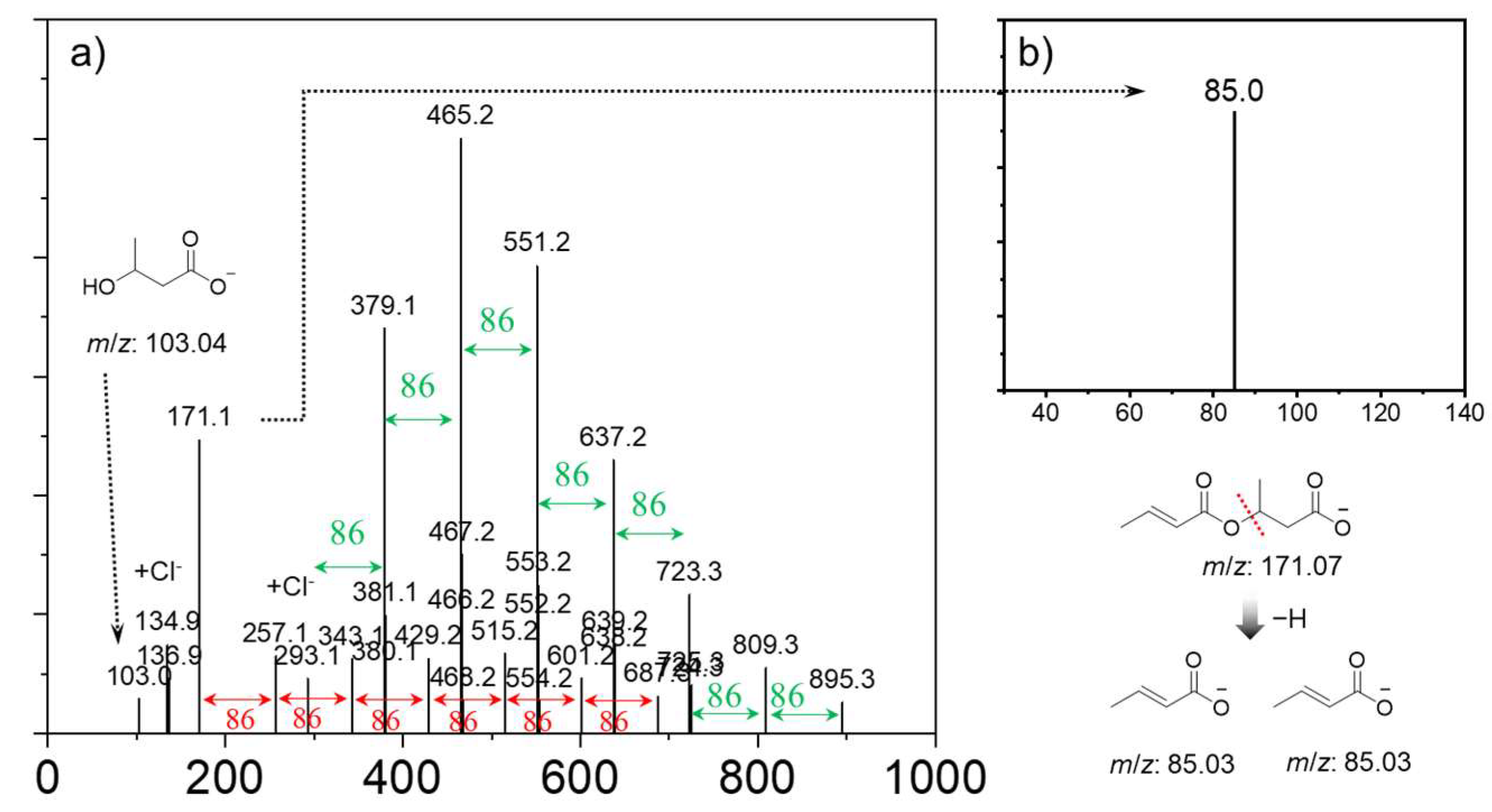
| Catalytic Systems | Conc. [i]0 (mol/L) 1 | Conc. [M]0 (mol/L) | Solvent | Temp. 2 (°C) | Time (h) | Mn (Da) | Yield (%) |
|---|---|---|---|---|---|---|---|
| Al(OiPr)3/DMAP | 0.073/0.82 | 7.48 | BnH 3 | 55 | 24 | 515 | 78.6 |
| Al(OiPr)3/Py | 0.073/0.82 | 7.48 | BnH | 55 | 24 | 465 | 75.2 |
| Py | 12.43 | 7.48 | Py 4 | 55 | 24 | 625 | 18.3 |
| DMAP | 0.82 | 7.48 | PEA 5 | 55 | 24 | 712 | 78.4 |
| Al(OiPr)3 | 0.073 | 7.48 | PEA | 55 | 24 | — | — |
| Al(OiPr)3/Py | 0.073/0.82 | 7.48 | PEA | 55 | 24 | 730 | 78.5 |
| Al(OiPr)3/DMAP | 0.073/0.82 | 7.48 | PEA | 55 | 24 | 821 | 85.3 |
| Virus Species Tested | Host Cells | Applied Concentration | Contact/Action Time | Average Lg TCID50/mL | Logarithm Reduction Value (KL) | Virus Inaction Ratio (%) |
|---|---|---|---|---|---|---|
| H1N1 (ATCC VR-1469) | MDCK | 0 mg/mL | 10 min | 5.58 ± 0.08 | — | — |
| 20 mg/mL | 10 min | 2.44 ± 0.12 | 3.14 | |||
| 0 mg/mL | 2 h | 5.71 ± 0.07 | — | — | ||
| 10 mg/mL | 2 h | 2.42 ± 0.01 | 3.30 | 99.95 | ||
| 0 mg/mL | 2 h | 5.74 ± 0.08 | — | — | ||
| 20 mg/mL | 2 h | <1.50 | >4.24 | >99.99 | ||
| H3N2 (ATCC VR-1679) | MDCK | 0 mg/mL | 10 min | 5.76 ± 0.07 | — | — |
| 20 mg/mL | 10 min | 2.45 ± 0.05 | 3.14 | |||
| 0 mg/mL | 2 h | 5.87 ± 0.06 | — | — | ||
| 10 mg/mL | 2 h | 2.44 ± 0.12 | 3.53 | 99.97 | ||
| 0 mg/mL | 2 h | 5.90 ± 0.10 | — | — | ||
| 20 mg/mL | 2 h | <1.50 | >4.40 | >99.99 | ||
| SARS-CoV-2 (IVCAS 6.7585) | Vero E6 | 0 mg/mL | 20 min | 7.18 ± 0.14 | — | — |
| 10 mg/mL | 20 min | 5.50 ± 0.01 | 1.32 | 95.16 | ||
| 0 mg/mL | 1.5 h | 6.63 ± 0.13 | — | — | ||
| 10 mg/mL | 1.5 h | 5.50 ± 0.02 | 1.13 | 92.57 | ||
| 20 mg/mL | 1.5 h | 5.50 ± 0.05 | 1.13 | 92.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Bao, Q.; So, P.-K.; Leung, S.L.; Leung, P.H.-M.; Tao, X. N, N-Dimethyl-4-Aminopyridine- and Aluminum Isopropoxide-Catalysed Ring-Opening Polymerizations of β-Butyrolactone for the Antimicrobial Oligohydroxybutyrate. Int. J. Mol. Sci. 2026, 27, 999. https://doi.org/10.3390/ijms27020999
Bao Q, So P-K, Leung SL, Leung PH-M, Tao X. N, N-Dimethyl-4-Aminopyridine- and Aluminum Isopropoxide-Catalysed Ring-Opening Polymerizations of β-Butyrolactone for the Antimicrobial Oligohydroxybutyrate. International Journal of Molecular Sciences. 2026; 27(2):999. https://doi.org/10.3390/ijms27020999
Chicago/Turabian StyleBao, Qi, Pui-Kin So, Siu Lun Leung, Polly Hang-Mei Leung, and Xiaoming Tao. 2026. "N, N-Dimethyl-4-Aminopyridine- and Aluminum Isopropoxide-Catalysed Ring-Opening Polymerizations of β-Butyrolactone for the Antimicrobial Oligohydroxybutyrate" International Journal of Molecular Sciences 27, no. 2: 999. https://doi.org/10.3390/ijms27020999
APA StyleBao, Q., So, P.-K., Leung, S. L., Leung, P. H.-M., & Tao, X. (2026). N, N-Dimethyl-4-Aminopyridine- and Aluminum Isopropoxide-Catalysed Ring-Opening Polymerizations of β-Butyrolactone for the Antimicrobial Oligohydroxybutyrate. International Journal of Molecular Sciences, 27(2), 999. https://doi.org/10.3390/ijms27020999






