Neuroprotection in Diabetes Retinal Disease: An Unmet Medical Need
Abstract
1. Introduction
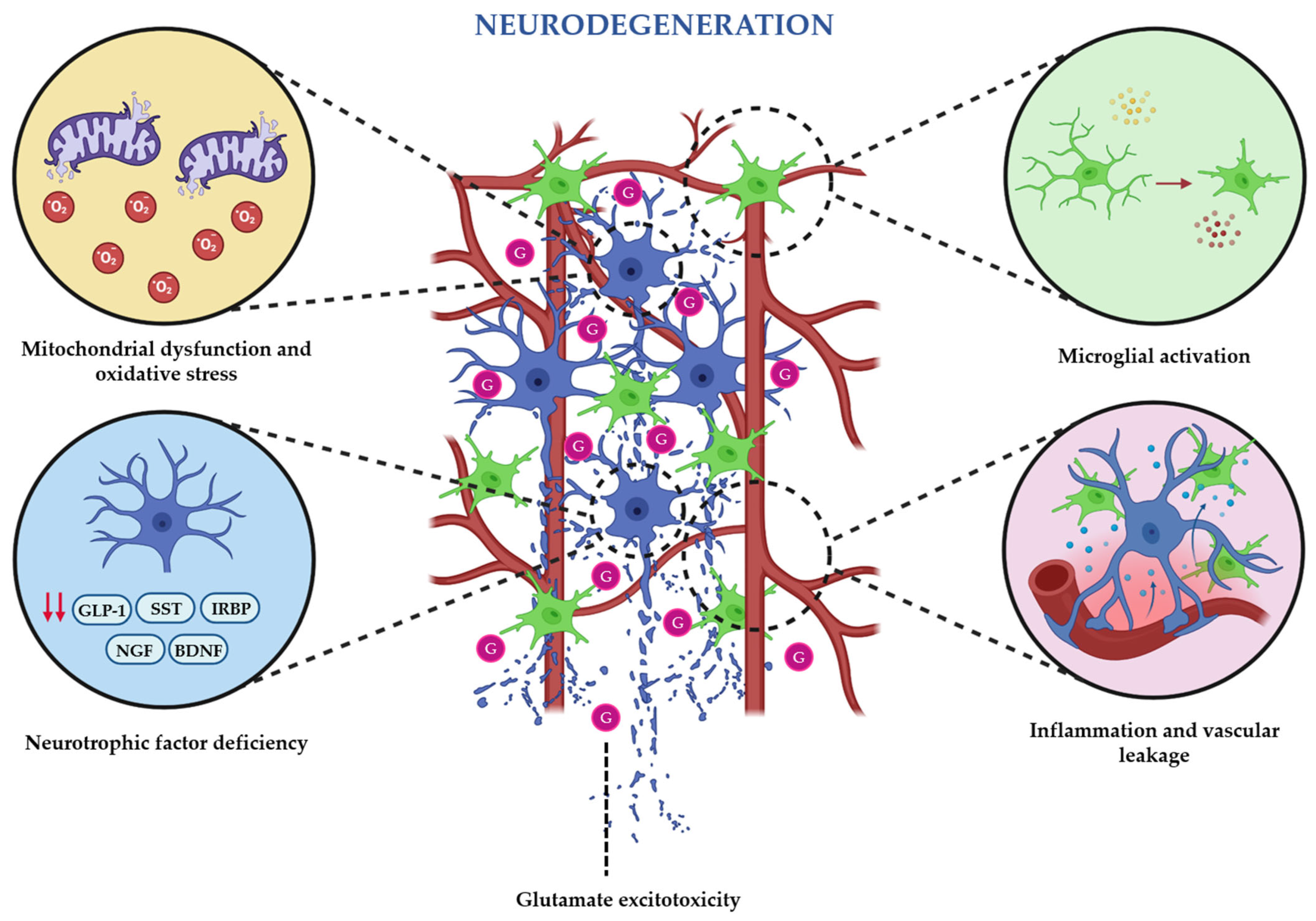
2. Pathogenic Mechanisms of Diabetes-Induced Retinal Neurodegeneration
2.1. Hyperglycemia: Underlying Mechanism in Early Neuronal Impairment
- -
- PKC pathway: When glyceraldehyde-3-phosphate levels increase, diacylglycerol (DAG) synthesis is enhanced activating PKC isoforms (α, β, δ, ε). In neurons, PKC dysregulation alters ion channel activity, neurotransmitter release, and calcium homeostasis, contributing to excitotoxic signaling and neuronal death. PKC-driven oxidative imbalance and pro-inflammatory signaling also compromise neuron–glia communication [29,30].
- -
- Hexosamine pathway: When fructose-6-phosphate is accumulated, it increases its flux into the hexosamine pathway, which produces an excess of UDP-N-acetylglucosamine. This overproduction leads to abnormal glycosylation of neuronal proteins and transcription factors, consequently disrupting normal gene expression and synaptic protein function. The hyperactivation of the hexosamine pathway has been associated with an increase in ROS levels, mitochondrial impairment, and reduced neuronal survival under stress conditions [29,31].
- -
- Polyol pathway: Hexokinase enzyme saturates due to the hyperglycemic state, and aldose reductase converts the excess of glucose into sorbitol by oxidizing nicotinamide adenine dinucleotide (NAD+/NADH) phosphate (NADPH) to NADP+ [32]. Sorbitol is then metabolized by sorbitol dehydrogenase to fructose using NAD+ as cofactor. Sorbitol accumulation causes osmotic stress [33], while NADPH reduction impairs glutathione regeneration and increases oxidative stress. Excessive sorbitol oxidation raises the NADH/NAD+ ratio, which inhibits glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and promotes DAG-mediated PKC activation and AGE formation [34]. Excess NADH can also fuel NADH oxidase, increasing ROS generation [28], while fructose metabolism produces potent glycation agents that further contribute to AGE [34]. Neurons express aldose reductase (particularly retinal ganglion cells), making them highly susceptible to polyol pathway activation [33].
- -
- AGEs pathway: Chronic hyperglycemia promotes the non-enzymatic glycation of neuronal proteins and lipids. AGEs interact with RAGE, which is expressed in ganglion cells and glia, and activates oxidative and inflammatory cascades that impair neuronal viability. In Müller cells, AGEs-RAGE signaling further compromises neuronal support by promoting VEGF release, inflammation, and gliosis [glial fibrillary acidic protein (GFAP) upregulation], aggravating neurodegeneration [28,35,36,37].
2.2. Oxidative Stress and Mitochondrial Dysfunction
2.3. Glutamate Excitotoxicity and Neurotransmitter Imbalance
2.4. Inflammation and Microglial Activation
2.5. Neurotrophic Factor Deficiency
2.6. Vascular–Neuronal Interactions (Neurovascular Unit Dysfunction)
3. Retinal Phenotyping in Diabetic Patients
4. Therapeutic Strategies Based on Neuroprotection
4.1. New Emerging Neuroprotective Agents
4.1.1. Antioxidants and Mitochondrial Protectors
4.1.2. Therapeutic Approaches Targeting the Reduction in Excitotoxicity and the Preservation of Synapses
4.1.3. Anti-Inflammatory and Immunomodulatory Agents
4.1.4. Vasoactive and Neurovascular Protectors
4.1.5. Therapies Based on Neurotrophic and Growth Factors
4.2. Novel Delivery Routes
4.3. Personalized Medicine: Patient Stratification and Targeted Therapies
5. Future Perspectives and Unmet Needs
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | Advanced Glycation End-products |
| ALA | α-Lipoic Acid |
| ATP | Adenosine Triphosphate |
| BDNF | Brain-Derived Neurotrophic Factor |
| BRB | Blood–Retinal Barrier |
| CDP-choline | Cytidine Diphosphate Choline |
| CNTF | Ciliary Neurotrophic Factor |
| COX | Cyclooxygenase |
| CREB | cAMP Response Element-Binding Protein |
| DAG | Diacylglycerol |
| DME | Diabetic Macular Edema |
| DPP-4 | Dipeptidyl Peptidase-4 |
| DR | Diabetic Retinopathy |
| EPO | Erythropoietin |
| ER | Endoplasmic Reticulum |
| ERG | Electroretinography |
| ETDRS | Early Treatment of Diabetic Retinopathy Study |
| EUROCONDOR | European Consortium for Early Treatment of Diabetic Retinopathy |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| GDNF | Glial Cell Line-Derived Neurotrophic Factor |
| GFAP | Glial Fibrillary Acidic Protein |
| GLAST | Glutamate Aspartate Transporter 1 |
| GLP-1 | Glucagon-Like Peptide-1 |
| GLP-1R | Glucagon-Like Peptide-1 Receptor |
| ICDR | International Clinical Diabetic Retinopathy |
| IL-1β | Interleukin-1 Beta |
| IRBP | Interstitial Retinol-Binding Protein |
| JAK/STAT | Janus Kinase/Signal Transducer and Activator of Transcription |
| NMDA | N-Methyl-D-Aspartate |
| NOX | NADPH Oxidase |
| NOX2 | NADPH Oxidase 2 |
| NRF2 | Nuclear Factor Erythroid 2–Related Factor 2 |
| NVU | Neurovascular Unit |
| OCT | Optical Coherence Tomography |
| PEDF | Pigment Epithelium-Derived Factor |
| PI3K/Akt | Phosphoinositide 3-Kinase/Protein Kinase B |
| PKC | Protein Kinase C |
| PPAR-α | Peroxisome Proliferator-Activated Receptor Alpha |
| RAC1 | Ras-Related C3 Botulinum Toxin Substrate 1 |
| RAGE | Receptor for Advanced Glycation End-products |
| RD | Retinal Disease |
| RGCs | Retinal Ganglion Cells |
| RPE | Retinal Pigment Epithelium |
| ROS | Reactive Oxygen Species |
| SkQ1 | Mitochondria-Targeted Antioxidant (Plastoquinone Derivative) |
| SOCS1 | Suppressor of Cytokine Signaling 1 |
| SST | Somatostatin |
| TNF-α | Tumor Necrosis Factor Alpha |
| UDP | Uridine Diphosphate |
| VEGF | Vascular Endothelial Growth Factor |
References
- Ting, D.S.; Cheung, G.C.; Wong, T.Y. Diabetic retinopathy: Global prevalence, major risk factors, screening practices and public health challenges: A review. Clin. Exp. Ophthalmol. 2016, 44, 260–277. [Google Scholar] [CrossRef]
- Bourne, R.R.A.; Jonas, J.B.; Bron, A.M.; Cicinelli, M.V.; Das, A.; Flaxman, S.R.; Friedman, D.S.; E Keeffe, J.; Kempen, J.H.; Leasher, J.; et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: Magnitude, temporal trends and projections. Br. J. Ophthalmol. 2018, 102, 575–585. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.J.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef]
- van Dijk, H.W.; Verbraak, F.D.; Kok, P.H.; Stehouwer, M.; Garvin, M.K.; Sonka, M.; DeVries, J.H.; Schlingemann, R.O.; Abràmoff, M.D. Early neurodegeneration in the retina of type 2 diabetic patients. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2715–2719, Erratum in Investig. Ophthalmol. Vis. Sci. 2012, 53, 3269. https://doi.org/10.1167/iovs.11-8997a. [Google Scholar] [CrossRef]
- Nian, S.; Lo, A.C.Y.; Mi, Y.; Ren, K.; Yang, D. Neurovascular unit in diabetic retinopathy: Pathophysiological roles and potential therapeutical targets. Eye Vis. 2021, 8, 15. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, H.; Su, S.B. Neuroinflammatory responses in diabetic retinopathy. J. Neuroinflamm. 2015, 12, 141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, M.Y.; Yiang, G.T.; Lai, T.T.; Li, C.J. The Oxidative Stress and Mitochondrial Dysfunction during the Pathogenesis of Diabetic Retinopathy. Oxid. Med. Cell Longev. 2018, 2018, 3420187. [Google Scholar] [CrossRef]
- Sinclair, S.H.; Schwartz, S.S. Diabetic Retinopathy—An Underdiagnosed and Undertreated Inflammatory, Neuro-Vascular Complication of Diabetes. Front. Endocrinol. 2019, 10, 843. [Google Scholar] [CrossRef]
- Vujosevic, S.; Midena, E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Müller cells alterations. J. Diabetes Res. 2013, 2013, 905058. [Google Scholar] [CrossRef]
- Chhablani, J.; Sharma, A.; Goud, A.; Peguda, H.K.; Rao, H.L.; Begum, V.U.; Barteselli, G. Neurodegeneration in Type 2 Diabetes: Evidence From Spectral-Domain Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6333–6338. [Google Scholar] [CrossRef]
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef]
- Madeira, M.H.; Marques, I.P.; Ferreira, S.; Tavares, D.; Santos, T.; Santos, A.R.; Figueira, J.; Lobo, C.; Cunha-Vaz, J. Retinal Neurodegeneration in Different Risk Phenotypes of Diabetic Retinal Disease. Front. Neurosci. 2021, 15, 800004. [Google Scholar] [CrossRef]
- Hernández, C.; Dal Monte, M.; Simó, R.; Casini, G. Neuroprotection as a Therapeutic Target for Diabetic Retinopathy. J. Diabetes Res. 2016, 2016, 9508541. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, B. Retinal Cell Damage in Diabetic Retinopathy. Cells 2023, 12, 1342. [Google Scholar] [CrossRef]
- He, W.; Tang, P.; Lv, H. Targeting oxidative stress in diabetic retinopathy: Mechanisms, pathology, and novel treatment approaches. Front. Immunol. 2025, 16, 1571576. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, Z.; Li, Y.; Liu, S.; Hu, P.; Luo, E. Advanced glycation end products and reactive oxygen species: Uncovering the potential role of ferroptosis in diabetic complications. Mol. Med. 2024, 30, 141. [Google Scholar] [CrossRef]
- Rana, D.; Dhankhar, S.; Chauhan, R.; Saini, M.; Singh, R.; Kumar, P.; Singh, T.G.; Chauhan, S.; Devi, S. Targeting neurotrophic dysregulation in diabetic retinopathy: A novel therapeutic avenue. Mol. Biol. Rep. 2025, 52, 570. [Google Scholar] [CrossRef] [PubMed]
- Callan, A.; Heckman, J.; Tah, G.; Lopez, S.; Valdez, L.; Tsin, A. VEGF in Diabetic Retinopathy and Age-Related Macular Degeneration. Int. J. Mol. Sci. 2025, 26, 4992. [Google Scholar] [CrossRef]
- Gupta, N.; Mansoor, S.; Sharma, A.; Sapkal, A.; Sheth, J.; Falatoonzadeh, P.; Kuppermann, B.; Kenney, M. Diabetic retinopathy and VEGF. Open Ophthalmol. J. 2013, 7, 4–10. [Google Scholar] [CrossRef]
- Oshitari, T. Neurovascular Impairment and Therapeutic Strategies in Diabetic Retinopathy. Int. J. Environ. Res. Public Health 2021, 19, 439. [Google Scholar] [CrossRef]
- Hurley, J.B.; Lindsay, K.J.; Du, J. Glucose, lactate, and shuttling of metabolites in vertebrate retinas. J. Neurosci. Res. 2015, 93, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Petit, L.; Ma, S.; Cipi, J.; Cheng, S.Y.; Zieger, M.; Hay, N.; Punzo, C. Aerobic Glycolysis Is Essential for Normal Rod Function and Controls Secondary Cone Death in Retinitis Pigmentosa. Cell Rep. 2018, 23, 2629–2642. [Google Scholar] [CrossRef] [PubMed]
- Rajala, A.; Bhat, M.A.; Teel, K.; Gopinadhan Nair, G.K.; Purcell, L.; Rajala, R.V.S. The function of lactate dehydrogenase A in retinal neurons: Implications to retinal degenerative diseases. PNAS Nexus 2023, 2, pgad038. [Google Scholar] [CrossRef]
- Kelly, K.; Wang, J.J.; Zhang, S.X. The unfolded protein response signaling and retinal Müller cell metabolism. Neural Regen. Res. 2018, 13, 1861–1870. [Google Scholar] [CrossRef]
- Fu, Z.; Kern, T.S.; Hellström, A.; Smith, L.E.H. Fatty acid oxidation and photoreceptor metabolic needs. J. Lipid Res. 2021, 62, 100035. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Yumnamcha, T.; Guerra, M.; Singh, L.P.; Ibrahim, A.S. Metabolic Dysregulation and Neurovascular Dysfunction in Diabetic Retinopathy. Antioxidants 2020, 9, 1244. [Google Scholar] [CrossRef]
- Geraldes, P.; King, G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef]
- Mathebula, S.D. Biochemical changes in diabetic retinopathy triggered by hyperglycaemia: A review. Afr. Vis. Eye Health 2018, 77, a439. [Google Scholar] [CrossRef]
- Safi, S.Z.; Qvist, R.; Kumar, S.; Batumalaie, K.; Ismail, I.S. Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. Biomed. Res. Int. 2014, 2014, 801269. [Google Scholar] [CrossRef]
- Lorenzi, M. The polyol pathway as a mechanism for diabetic retinopathy: Attractive, elusive, and resilient. Exp. Diabetes Res. 2007, 2007, 61038. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Ward, M.; Stitt, A.W. AGEs, RAGE, and diabetic retinopathy. Curr. Diabetes Rep. 2011, 11, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.H.; Kang, E.Y.; Lin, P.H.; Yu, B.B.; Wang, J.H.; Chen, V.; Wang, N.K. Mitochondria in Retinal Ganglion Cells: Unraveling the Metabolic Nexus and Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 8626. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.J.; Cascio, M.A.; Rosca, M.G. Diabetic Retinopathy: The Role of Mitochondria in the Neural Retina and Microvascular Disease. Antioxidants 2020, 9, 905. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mishra, M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim. Biophys. Acta 2015, 1852, 2474–2483. [Google Scholar] [CrossRef]
- Kowluru, R.A. Diabetic Retinopathy and NADPH Oxidase-2: A Sweet Slippery Road. Antioxidants 2021, 10, 783. [Google Scholar] [CrossRef]
- Sahajpal, N.; Kowluru, A.; Kowluru, R.A. The Regulatory Role of Rac1, a Small Molecular Weight GTPase, in the Development of Diabetic Retinopathy. J. Clin. Med. 2019, 8, 965. [Google Scholar] [CrossRef]
- Madonna, R.; Giovannelli, G.; Confalone, P.; Renna, F.V.; Geng, Y.J.; De Caterina, R. High glucose-induced hyperosmolarity contributes to COX-2 expression and angiogenesis: Implications for diabetic retinopathy. Cardiovasc. Diabetol. 2016, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Gubitosi-Klug, R.A.; Talahalli, R.; Du, Y.; Nadler, J.L.; Kern, T.S. 5-Lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes 2008, 57, 1387–1393. [Google Scholar] [CrossRef]
- Pacher, P.; Nivorozhkin, A.; Szabó, C. Therapeutic effects of xanthine oxidase inhibitors: Renaissance half a century after the discovery of allopurinol. Pharmacol. Rev. 2006, 58, 87–114. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.; Bogdanov, P.; Sampedro, J.; Huerta, J.; Simó, R.; Hernández, C. Beneficial Effects of Glucagon-Like Peptide-1 (GLP-1) in Diabetes-Induced Retinal Abnormalities: Involvement of Oxidative Stress. Antioxidants 2020, 9, 846. [Google Scholar] [CrossRef] [PubMed]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Albert-Garay, J.S.; Riesgo-Escovar, J.R.; Salceda, R. High glucose concentrations induce oxidative stress by inhibiting Nrf2 expression in rat Müller retinal cells in vitro. Sci. Rep. 2022, 12, 1261. [Google Scholar] [CrossRef]
- Hu, W.; Tu, Y.; Tan, J.; Lin, Y.; Wang, Y.; Zhou, Q. Global research trends on endoplasmic reticulum stress in retinal diseases from 2000 to 2024. Int. Ophthalmol. 2025, 45, 210. [Google Scholar] [CrossRef]
- Ishikawa, M. Abnormalities in glutamate metabolism and excitotoxicity in the retinal diseases. Scientifica 2013, 2013, 528940. [Google Scholar] [CrossRef]
- Li, Q.; Puro, D.G. Diabetes-induced dysfunction of the glutamate transporter in retinal Müller cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3109–3116. [Google Scholar]
- Lau, J.C.; Kroes, R.A.; Moskal, J.R.; Linsenmeier, R.A. Diabetes changes expression of genes related to glutamate neurotransmission and transport in the Long-Evans rat retina. Mol. Vis. 2013, 19, 1538–1553. [Google Scholar]
- Christensen, I.; Lu, B.; Yang, N.; Huang, K.; Wang, P.; Tian, N. The Susceptibility of Retinal Ganglion Cells to Glutamatergic Excitotoxicity Is Type-Specific. Front. Neurosci. 2019, 13, 219. [Google Scholar] [CrossRef] [PubMed]
- VanGuilder, H.D.; Brucklacher, R.M.; Patel, K.; Ellis, R.W.; Freeman, W.M.; Barber, A.J. Diabetes downregulates presynaptic proteins and reduces basal synapsin I phosphorylation in rat retina. Eur. J. Neurosci. 2008, 28, 1–11. [Google Scholar] [CrossRef]
- Zhang, L.; Ino-ue, M.; Dong, K.; Yamamoto, M. Retrograde axonal transport impairment of large- and medium-sized retinal ganglion cells in diabetic rat. Curr. Eye Res. 2000, 20, 131–136. [Google Scholar] [CrossRef]
- Robinson, W.F.; VanGuilder, H.D.; D’Cruz, T.S.; El-Remessy, A.B.; Barber, A.J. Synapsin 1 protein expression and phosphorylation are compromised by diabetes in rodent and human retinas. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4920. [Google Scholar]
- Ly, A.; Scheerer, M.F.; Zukunft, S.; Muschet, C.; Merl, J.; Adamski, J.; de Angelis, M.H.; Neschen, S.; Hauck, S.M.; Ueffing, M. Retinal proteome alterations in a mouse model of type 2 diabetes. Diabetologia 2014, 57, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Kinuthia, U.M.; Wolf, A.; Langmann, T. Microglia and Inflammatory Responses in Diabetic Retinopathy. Front. Immunol. 2020, 11, 564077. [Google Scholar] [CrossRef] [PubMed]
- Srejovic, J.V.; Muric, M.D.; Jakovljevic, V.L.; Srejovic, I.M.; Sreckovic, S.B.; Petrovic, N.T. Molecular and Cellular Mechanisms Involved in the Pathophysiology of Retinal Vascular Disease-Interplay Between Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 11850. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Xia, Y.; Luo, Q.; Chen, J.; Huang, C.; Jahangir, A.; Pan, T.; Wei, X.; Liu, W.; Chen, Z. Retinal Astrocytes and Microglia Activation in Diabetic Retinopathy Rhesus Monkey Models. Curr. Eye Res. 2022, 47, 297–303. [Google Scholar] [CrossRef]
- Simó, R.; Hernández, C. European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Neurodegeneration in the diabetic eye: New insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014, 25, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Xie, H.; Zhang, C.; Wang, T.; Tian, H.; Lu, L.; Xu, J.; Xu, G.; Liu, L.; Zhang, J. Enhancing fractalkine/CX3CR1 signalling pathway can reduce neuroinflammation by attenuating microglia activation in experimental diabetic retinopathy. J. Cell Mol. Med. 2022, 26, 1229–1244. [Google Scholar] [CrossRef]
- Yuan, T.; Dong, L.; Pearsall, E.A.; Zhou, K.; Cheng, R.; Ma, J.X. The Protective Role of Microglial PPARα in Diabetic Retinal Neurodegeneration and Neurovascular Dysfunction. Cells 2022, 11, 3869. [Google Scholar] [CrossRef]
- Simó, R.; Simó-Servat, O.; Bogdanov, P.; Hernández, C. Neurovascular Unit: A New Target for Treating Early Stages of Diabetic Retinopathy. Pharmaceutics 2021, 13, 1320. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Bogdanov, P.; Corraliza, L.; García-Ramírez, M.; Solà-Adell, C.; Arranz, J.A.; Arroba, A.I.; Valverde, A.M.; Simó, R. Topical Administration of GLP-1 Receptor Agonists Prevents Retinal Neurodegeneration in Experimental Diabetes. Diabetes 2016, 65, 172–187. [Google Scholar] [CrossRef]
- Garcia-Ramírez, M.; Hernández, C.; Villarroel, M.; Canals, F.; Alonso, M.A.; Fortuny, R.; Masmiquel, L.; Navarro, A.; García-Arumí, J.; Simó, R. Interphotoreceptor retinoid-binding protein (IRBP) is downregulated at early stages of diabetic retinopathy. Diabetologia 2009, 52, 2633–2641. [Google Scholar] [CrossRef]
- Barnstable, C.J.; Tombran-Tink, J. Neuroprotective and antiangiogenic actions of PEDF in the eye: Molecular targets and therapeutic potential. Prog. Retin. Eye Res. 2004, 23, 561–577. [Google Scholar] [CrossRef]
- Carrasco, E.; Hernández, C.; Miralles, A.; Huguet, P.; Farrés, J.; Simó, R. Lower somatostatin expression is an early event in diabetic retinopathy and is associated with retinal neurodegeneration. Diabetes Care 2007, 30, 2902–2908. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, F.; Ghosh, D. Focus on molecules: Interphotoreceptor retinoidbinding protein (IRBP). Exp. Eye Res. 2008, 86, 169–170. [Google Scholar] [CrossRef] [PubMed]
- den Hollander, A.I.; McGee, T.L.; Ziviello, C.; Banfi, S.; Dryja, T.P.; Gonzalez-Fernandez, F.; Ghosh, D.; Berson, E.L. A homozygous missense mutation in the IRBP gene (RBP3) associated with autosomal recessive retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1864–1872. [Google Scholar] [CrossRef]
- Mysona, B.A.; Shanab, A.Y.; Elshaer, S.L.; El-Remessy, A.B. Nerve growth factor in diabetic retinopathy: Beyond neurons. Expert Rev. Ophthalmol. 2014, 9, 99–107. [Google Scholar] [CrossRef]
- Afarid, M.; Namvar, E.; Sanie-Jahromi, F. Diabetic Retinopathy and BDNF: A Review on Its Molecular Basis and Clinical Applications. J. Ophthalmol. 2020, 2020, 1602739. [Google Scholar] [CrossRef]
- Kalhoro, R.; Raffad; Durrani, M.U.; Munim, A.; Jamil, N.; Ali, M.I.; Shah, M.U. Clinicopathological Assessments of Brain-Derived Neurotrophic Factor BDNF Physiology in Diabetic Retinopathy: A Systematic Review. Pak. J. Med. Dent. 2025, 14, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Gui, F.; You, Z.; Fu, S.; Wu, H.; Zhang, Y. Endothelial Dysfunction in Diabetic Retinopathy. Front. Endocrinol. 2020, 11, 591. [Google Scholar] [CrossRef]
- Santiago, A.R.; Boia, R.; Aires, I.D.; Ambrósio, A.F.; Fernandes, R. Sweet Stress: Coping with Vascular Dysfunction in Diabetic Retinopathy. Front. Physiol. 2018, 9, 820. [Google Scholar] [CrossRef] [PubMed]
- Hammes, H.P.; Lin, J.; Renner, O.; Shani, M.; Lundqvist, A.; Betsholtz, C.; Brownlee, M.; Deutsch, U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 2002, 51, 3107–3112. [Google Scholar] [CrossRef]
- Leal, E.C.; Martins, J.; Voabil, P.; Liberal, J.; Chiavaroli, C.; Bauer, J.; Cunha-Vaz, J.; Ambrósio, A.F. Calcium dobesilate inhibits the alterations in tight junction proteins and leukocyte adhesion to retinal endothelial cells induced by diabetes. Diabetes 2010, 59, 2637–2645. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Behzadian, M.A.; Abou-Mohamed, G.; Franklin, T.; Caldwell, R.W.; Caldwell, R.B. Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. Am. J. Pathol. 2003, 162, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Aldosari, D.I.; Malik, A.; Alhomida, A.S.; Ola, M.S. Implications of Diabetes-Induced Altered Metabolites on Retinal Neurodegeneration. Front. Neurosci. 2022, 16, 938029. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Gardner, T.W.; Abcouwer, S.F. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1156–1163. [Google Scholar] [CrossRef]
- Yang, Z.; Tan, T.E.; Shao, Y.; Wong, T.Y.; Li, X. Classification of diabetic retinopathy: Past, present and future. Front. Endocrinol. 2022, 13, 1079217. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology 1991, 98, 766–785. [Google Scholar] [CrossRef]
- Wilkinson, C.P.; Ferris, F.L., 3rd; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Zur, D.; Iglicki, M.; Busch, C.; Invernizzi, A.; Mariussi, M.; Loewenstein, A. International Retina Group. OCT Biomarkers as Functional Outcome Predictors in Diabetic Macular Edema Treated with Dexamethasone Implant. Ophthalmology 2018, 125, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.K.; Lin, M.M.; Lammer, J.; Prager, S.; Sarangi, R.; Silva, P.S.; Aiello, L.P. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014, 132, 1309–1316. [Google Scholar] [CrossRef]
- Muftuoglu, I.K.; Mendoza, N.; Gaber, R.; Alam, M.; You, Q.; Freeman, W.R. INTEGRITY OF OUTER RETINAL LAYERS AFTER RESOLUTION OF CENTRAL INVOLVED DIABETIC MACULAR EDEMA. Retina 2017, 37, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Markan, A.; Agarwal, A.; Arora, A.; Bazgain, K.; Rana, V.; Gupta, V. Novel imaging biomarkers in diabetic retinopathy and diabetic macular edema. Ther. Adv. Ophthalmol. 2020, 12, 2515841420950513. [Google Scholar] [CrossRef]
- Udaondo, P.; Hernández, C.; Briansó-Llort, L.; García-Delpech, S.; Simó-Servat, O.; Simó, R. Usefulness of Liquid Biopsy Biomarkers from Aqueous Humor in Predicting Anti-VEGF Response in Diabetic Macular Edema: Results of a Pilot Study. J. Clin. Med. 2019, 8, 1841. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.R.; Ribeiro, L.; Bandello, F.; Lattanzio, R.; Egan, C.; Frydkjaer-Olsen, U.; García-Arumí, J.; Gibson, J.; Grauslund, J.; Harding, S.P.; et al. Functional and structural findings of neurodegeneration in early stages of diabetic retinopathy: Cross-sectional analyses of baseline data of the EUROCONDOR project. Diabetes 2017, 66, 2503–2510. [Google Scholar] [CrossRef]
- Simó, R.; Hernández, C.; Porta, M.; Bandello, F.; Grauslund, J.; Harding, S.P.; Aldington, S.J.; Egan, C.; Frydkjaer-Olsen, U.; Garcia-Arumi, J.; et al. Effects of Topically Administered Neuroprotective Drugs in Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. Diabetes 2019, 68, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Channa, R.; Wolf, R.M.; Simo, R.; Brigell, M.; Fort, P.; Curcio, C.; Lynch, S.; Verbraak, F.; Abramoff, M.D.; Brigell, M.; et al. Diabetic Retinal Neurodegeneration and Macular Edema working group of the Mary Tyler Moore Vision Initiative’s Diabetic Retinal Disease Staging Update Project. A New Approach to Staging Diabetic Eye Disease: Staging of Diabetic Retinal Neurodegeneration and Diabetic Macular Edema. Ophthalmol. Sci. 2023, 4, 100420. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Hysi, P.G.; Khawaja, A.P.; Mahroo, O.A.; Xu, Z.; Hammond, C.J.; Foster, P.J.; Welikala, R.A.; Barman, S.A.; Whincup, P.H.; et al. UK Biobank Eye and Vision Consortium. GWAS on retinal vasculometry phenotypes. PLoS Genet. 2023, 19, e1010583. [Google Scholar] [CrossRef]
- Jackson, V.E.; Wu, Y.; Bonelli, R.; Owen, J.P.; Scott, L.W.; Farashi, S.; Kihara, Y.; Gantner, M.L.; Egan, C.; Williams, K.M.; et al. Multi-omic spatial effects on high-resolution AI-derived retinal thickness. Nat. Commun. 2025, 16, 1317. [Google Scholar] [CrossRef] [PubMed]
- Villarroel, M.; Ciudin, A.; Hernández, C.; Simó, R. Neurodegeneration: An early event of diabetic retinopathy. World J. Diabetes 2010, 1, 57–64. [Google Scholar] [CrossRef]
- Pearsall, E.A.; Cheng, R.; Matsuzaki, S.; Zhou, K.; Ding, L.; Ahn, B.; Kinter, M.; Humphries, K.M.; Quiambao, A.B.; A Farjo, R.; et al. Neuroprotective effects of PPARα in retinopathy of type 1 diabetes. PLoS ONE 2019, 14, e0208399. [Google Scholar] [CrossRef]
- Wubben, T.J.; Besirli, C.G.; Johnson, M.W.; Zacks, D.N. Retinal Neuroprotection: Overcoming the Translational Roadblocks. Am. J. Ophthalmol. 2018, 192, xv–xxii. [Google Scholar] [CrossRef]
- Yang, Q.; Yasvoina, M.; Olvera-Barrios, A.; Mendes, J.; Zhu, M.; Egan, C.; Tufail, A.; Fruttiger, M. Deciphering the Connection Between Microvascular Damage and Neurodegeneration in Early Diabetic Retinopathy. Diabetes 2024, 73, 1883–1894. [Google Scholar] [CrossRef]
- Sun, J.K.; Aiello, L.P.; Abràmoff, M.D.; Antonetti, D.A.; Dutta, S.; Pragnell, M.; Levine, S.R.; Gardner, T.W. Updating the Staging System for Diabetic Retinal Disease. Ophthalmology 2021, 128, 490–493. [Google Scholar] [CrossRef]
- Sachdeva, M.M. Retinal Neurodegeneration in Diabetes: An Emerging Concept in Diabetic Retinopathy. Curr. Diabetes Rep. 2021, 21, 65. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Odenbach, S. Effect of long-term administration of alpha-lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes 2004, 53, 3233–3238. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Bierhaus, A.; Bugert, P.; Dietrich, N.; Feng, Y.; Vom Hagen, F.; Nawroth, P.; Brownlee, M.; Hammes, H.-P. Effect of R-(+)-alpha-lipoic acid on experimental diabetic retinopathy. Diabetologia 2006, 49, 1089–1096. [Google Scholar] [CrossRef]
- Markovets, A.M.; Fursova, A.Z.; Kolosova, N.G. Therapeutic action of the mitochondria-targeted antioxidant SkQ1 on retinopathy in OXYS rats linked with improvement of VEGF and PEDF gene expression. PLoS ONE 2011, 6, e21682. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Walton, D.A.; Plafker, K.S.; Boulton, M.E.; Plafker, S.M. Sulforaphane recovers cone function in an Nrf2-dependent manner in middle-aged mice undergoing RPE oxidative stress. Mol. Vis. 2022, 28, 378–393. [Google Scholar]
- Grauslund, J.; Frydkjaer-Olsen, U.; Peto, T.; Fernández-Carneado, J.; Ponsati, B.; Hernández, C. EUROCONDOR. Topical Treatment With Brimonidine and Somatostatin Causes Retinal Vascular Dilation in Patients with Early Diabetic Retinopathy From the EUROCONDOR. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2257–2262. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Centofanti, M.; Ziccardi, L.; Tanga, L.; Michelessi, M.; Roberti, G. Treatment with citicoline eye drops enhances retinal function and neural conduction along the visual pathways in open angle glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1327–1340. [Google Scholar] [CrossRef]
- Parisi, V.; Oddone, F.; Ziccardi, L.; Roberti, G.; Coppola, G.; Manni, G. Citicoline and Retinal Ganglion Cells: Effects on Morphology and Function. Curr. Neuropharmacol. 2018, 16, 919–932. [Google Scholar] [CrossRef]
- Bogdanov, P.; Sampedro, J.; Solà-Adell, C.; Simó-Servat, O.; Russo, C.; Varela-Sende, L.; Simó, R.; Hernández, C. Effects of Liposomal Formulation of Citicoline in Experimental Diabetes-Induced Retinal Neurodegeneration. Int. J. Mol. Sci. 2018, 19, 2458. [Google Scholar] [CrossRef]
- Kusari, J.; Zhou, S.; Padillo, E.; Clarke, K.G.; Gil, D.W. Effect of memantine on neuroretinal function and retinal vascular changes of streptozotocin-induced diabetic rats. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5152–5159. [Google Scholar] [CrossRef]
- Hernández, C.; Bogdanov, P.; Gómez-Guerrero, C.; Sampedro, J.; Solà-Adell, C.; Espejo, C.; García-Ramírez, M.; Prieto, I.; Egido, J.; Simó, R. SOCS1-Derived Peptide Administered by Eye Drops Prevents Retinal Neuroinflammation and Vascular Leakage in Experimental Diabetes. Int. J. Mol. Sci. 2019, 20, 3615. [Google Scholar] [CrossRef]
- Scholz, R.; Sobotka, M.; Caramoy, A.; Stempfl, T.; Moehle, C.; Langmann, T. Minocycline counter-regulates pro-inflammatory microglia responses in the retina and protects from degeneration. J. Neuroinflamm. 2015, 12, 209. [Google Scholar] [CrossRef]
- Paterniti, I.; Di Paola, R.; Campolo, M.; Siracusa, R.; Cordaro, M.; Bruschetta, G.; Tremolada, G.; Maestroni, A.; Bandello, F.; Esposito, E.; et al. Palmitoylethanolamide treatment reduces retinal inflammation in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2015, 769, 313–323. [Google Scholar] [CrossRef]
- Sugiyama, T. Role of P2X7 receptors in the development of diabetic retinopathy. World J. Diabetes 2014, 5, 141–145. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Wu, S.; Jin, J.; Li, W.; Wang, N. Calcium dobesilate for diabetic retinopathy: A systematic review and meta-analysis. Sci. China Life Sci. 2015, 58, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, P.; Simó-Servat, O.; Sampedro, J.; Solà-Adell, C.; Garcia-Ramírez, M.; Ramos, H.; Guerrero, M.; Suñé-Negre, J.M.; Ticó, J.R.; Montoro, B.; et al. Topical Administration of Bosentan Prevents Retinal Neurodegeneration in Experimental Diabetes. Int. J. Mol. Sci. 2018, 19, 3578. [Google Scholar] [CrossRef] [PubMed]
- Aizu, Y.; Katayama, H.; Takahama, S.; Hu, J.; Nakagawa, H.; Oyanagi, K. Topical instillation of ciliary neurotrophic factor inhibits retinal degeneration in streptozotocin-induced diabetic rats. Neuroreport 2003, 14, 2067–2071. [Google Scholar] [CrossRef]
- Liu, Y.; Leo, L.F.; McGregor, C.; Grivitishvili, A.; Barnstable, C.J.; Tombran-Tink, J. Pigment epithelium-derived factor (PEDF) peptide eye drops reduce inflammation, cell death and vascular leakage in diabetic retinopathy in Ins2(Akita) mice. Mol. Med. 2012, 18, 1387–1401. [Google Scholar] [CrossRef]
- Zerbini, G.; Maestroni, S.; Leocani, L.; Mosca, A.; Godi, M.; Paleari, R.; Belvedere, A.; Gabellini, D.; Tirassa, P.; Castoldi, V.; et al. Topical nerve growth factor prevents neurodegenerative and vascular stages of diabetic retinopathy. Front. Pharmacol. 2022, 13, 1015522. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Xu, H.; Wang, F.; Xu, G.; Sinha, D.; Wang, J.; Xu, J.-Y.; Tian, H.; Gao, F.; Li, W.; et al. Erythropoietin exerts a neuroprotective function against glutamate neurotoxicity in experimental diabetic retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8208–8222. [Google Scholar] [CrossRef]
- Sampedro, J.; Bogdanov, P.; Ramos, H.; Solà-Adell, C.; Turch, M.; Valeri, M.; Simó-Servat, O.; Lagunas, C.; Simó, R.; Hernández, C. New Insights into the Mechanisms of Action of Topical Administration of GLP-1 in an Experimental Model of Diabetic Retinopathy. J. Clin. Med. 2019, 8, 339. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, K.; Wang, Q.; Ruan, Y.; Zhang, Y.; Ye, W. Exendin-4 protects retinal cells from early diabetes in Goto-Kakizaki rats by increasing the Bcl-2/Bax and Bcl-xL/Bax ratios and reducing reactive gliosis. Mol. Vis. 2014, 20, 1557–1568. [Google Scholar]
- Ramos, H.; Bogdanov, P.; Sabater, D.; Huerta, J.; Valeri, M.; Hernández, C.; Simó, R. Neuromodulation Induced by Sitagliptin: A New Strategy for Treating Diabetic Retinopathy. Biomedicines 2021, 9, 1772. [Google Scholar] [CrossRef]
- Ramos, H.; Bogdanov, P.; Simó, R.; Deàs-Just, A.; Hernández, C. Transcriptomic Analysis Reveals That Retinal Neuromodulation Is a Relevant Mechanism in the Neuroprotective Effect of Sitagliptin in an Experimental Model of Diabetic Retinopathy. Int. J. Mol. Sci. 2022, 24, 571. [Google Scholar] [CrossRef]
- Ramos, H.; Augustine, J.; Karan, B.M.; Hernández, C.; Stitt, A.W.; Curtis, T.M.; Simó, R. Sitagliptin eye drops prevent the impairment of retinal neurovascular unit in the new Trpv2+/− rat model. J. Neuroinflamm. 2024, 21, 312. [Google Scholar] [CrossRef]
- Gholami, M.; Balajam, N.Z.; Rakhsham, S.; Sajjadi-Jazi, S.M.; Shafiee, G.; Heshmat, R. GLP-1 receptor agonists efficacy in managing comorbidities associated with diabetes mellitus: A narrative review. J. Diabetes Metab. Disord. 2025, 24, 92. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, N.; Lin, P.; Xing, Y.; Yang, N. Recent advances in the treatment and delivery system of diabetic retinopathy. Front. Endocrinol. 2024, 15, 1347864. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Pande, S.; Sagathia, V.; Ranch, K.; Beladiya, J.; Boddu, S.H.S.; Jacob, S.; Al-Tabakha, M.M.; Hassan, N.; Shahwan, M. Nanocarriers for the Delivery of Neuroprotective Agents in the Treatment of Ocular Neurodegenerative Diseases. Pharmaceutics 2023, 15, 837. [Google Scholar] [CrossRef] [PubMed]
- Khatun, A.; Hu, V.H. Safety around medicines for eye care. Community Eye Health 2021, 34, 19–21. [Google Scholar]
- Lampert, A.; Bruckner, T.; Haefeli, W.E.; Seidling, H.M. Improving eye-drop administration skills of patients—A multicenter parallel-group cluster-randomized controlled trial. PLoS ONE 2019, 14, e0212007. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.; Dal Monte, M.; Lulli, M.; Raffa, V.; Casini, G. Nanoparticle-Mediated Delivery of Neuroprotective Substances for the Treatment of Diabetic Retinopathy. Curr. Neuropharmacol. 2018, 16, 993–1003. [Google Scholar] [CrossRef]
- Rafael, D.; Guerrero, M.; Marican, A.; Arango, D.; Sarmento, B.; Ferrer, R.; Durán-Lara, E.F.; Clark, S.J.; Schwartz, S. Delivery Systems in Ocular Retinopathies: The Promising Future of Intravitreal Hydrogels as Sustained-Release Scaffolds. Pharmaceutics 2023, 15, 1484. [Google Scholar] [CrossRef]
- Kąpa, M.; Koryciarz, I.; Kustosik, N.; Jurowski, P.; Pniakowska, Z. Future Directions in Diabetic Retinopathy Treatment: Stem Cell Therapy, Nanotechnology, and PPARα Modulation. J. Clin. Med. 2025, 14, 683. [Google Scholar] [CrossRef]
- Jenkins, A.J.; Joglekar, M.V.; Hardikar, A.A.; Keech, A.C.; O’Neal, D.N.; Januszewski, A.S. Biomarkers in Diabetic Retinopathy. Rev. Diabet. Stud. 2015, 12, 159–195. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Simó, R. Neuroprotection in diabetic retinopathy. Curr. Diabetes Rep. 2012, 12, 329–337. [Google Scholar] [CrossRef]
- Micera, A.; Balzamino, B.O.; Di Zazzo, A.; Dinice, L.; Bonini, S.; Coassin, M. Biomarkers of Neurodegeneration and Precision Therapy in Retinal Disease. Front. Pharmacol. 2021, 11, 601647. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Chen, F.; Li, Y.; Zhou, R.; Wu, C.; Yu, H.; Lin, Z.; Shi, C.; Zheng, G.; et al. Visual acuity is correlated with ischemia and neurodegeneration in patients with early stages of diabetic retinopathy. Eye Vis. 2021, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Moore-Dotson, J.M.; Beckman, J.J.; Mazade, R.E.; Hoon, M.; Bernstein, A.S.; Romero-Aleshire, M.J.; Brooks, H.L.; Eggers, E.D. Early Retinal Neuronal Dysfunction in Diabetic Mice: Reduced Light-Evoked Inhibition Increases Rod Pathway Signaling. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1418–1430. [Google Scholar] [CrossRef]
- Ozawam, Y.; Kurihara, T.; Sasaki, M.; Ban, N.; Yuki, K.; Kubota, S.; Tsubota, K. Neural degeneration in the retina of the streptozotocin-induced type 1 diabetes model. Exp. Diabetes Res. 2011, 2011, 108328. [Google Scholar] [CrossRef] [PubMed]
- Bianco, L.; Arrigo, A.; Aragona, E.; Antropoli, A.; Berni, A.; Saladino, A.; Parodi, M.B.; Bandello, F. Neuroinflammation and neurodegeneration in diabetic retinopathy. Front. Aging Neurosci. 2022, 14, 937999. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Zheng, X.; Yang, M.M. New insights of potential biomarkers in diabetic retinopathy: Integrated multi-omic analyses. Front. Endocrinol. 2025, 16, 1595207. [Google Scholar] [CrossRef]
| Neuroprotective Agent | Primary Mechanism of Action | Evidence/Trial Status |
|---|---|---|
| α-Lipoic acid (ALA) | Antioxidant activity and mitochondrial function preservation | Preclinical |
| SkQ1 | Mitochondria-targeted antioxidant activity | Preclinical |
| Sulforaphane | Activation of endogenous antioxidant defenses (NRF2 pathway) | Preclinical |
| Brimonidine | Reduction in glutamate excitotoxicity and activation of neuronal survival signaling | Phase II/III clinical trial; no significant effect on primary endpoint |
| Citicoline (CDP-choline) | Membrane stabilization and support of synaptic and mitochondrial function | Preclinical; small exploratory human studies |
| Memantine | NMDA receptor antagonism; reduction in excitotoxicity | Preclinical |
| SOCS1-derived peptides | Inhibition of cytokine signaling and glial activation | Preclinical |
| Minocycline | Modulation of microglial activation and inflammatory signaling | Preclinical |
| Palmitoylethanolamide | PPAR-α-mediated anti-inflammatory signaling | Preclinical |
| P2X7 receptor inhibitors | Suppression of inflammasome-related signaling | Preclinical |
| Calcium dobesilate | Improvement of microvascular function and reduction in oxidative stress | Controlled trials; evidence on vascular outcomes, not designed for neuroprotection |
| Bosentan | Endothelin receptor antagonism and improvement of retinal perfusion | Preclinical |
| Somatostatin analogs | Restoration of neurotrophic signaling and modulation of neuronal activity | Phase II/III clinical trial; no significant effect on primary endpoint |
| CNTF | Neurotrophic support promoting retinal ganglion cell survival | Preclinical/early-phase clinical studies |
| BDNF | Neurotrophic support and synaptic maintenance | Preclinical |
| PEDF | Anti-angiogenic and neuroprotective activity | Preclinical |
| NGF | Neurotrophic support and neuronal survival | |
| Erythropoietin (EPO) | Anti-apoptotic and neuroprotective signaling | Preclinical |
| GLP-1 receptor agonists (topical) | Metabolic and neurotrophic signaling enhancing neuronal survival | Preclinical |
| DPP-4 inhibitors (topical) | Enhancement of endogenous GLP-1 signaling and neuroprotection | Preclinical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ramos, H.; Simó-Servat, O. Neuroprotection in Diabetes Retinal Disease: An Unmet Medical Need. Int. J. Mol. Sci. 2026, 27, 901. https://doi.org/10.3390/ijms27020901
Ramos H, Simó-Servat O. Neuroprotection in Diabetes Retinal Disease: An Unmet Medical Need. International Journal of Molecular Sciences. 2026; 27(2):901. https://doi.org/10.3390/ijms27020901
Chicago/Turabian StyleRamos, Hugo, and Olga Simó-Servat. 2026. "Neuroprotection in Diabetes Retinal Disease: An Unmet Medical Need" International Journal of Molecular Sciences 27, no. 2: 901. https://doi.org/10.3390/ijms27020901
APA StyleRamos, H., & Simó-Servat, O. (2026). Neuroprotection in Diabetes Retinal Disease: An Unmet Medical Need. International Journal of Molecular Sciences, 27(2), 901. https://doi.org/10.3390/ijms27020901






