Enhanced Qualities of High-Density Lipoproteins (HDLs) with Antioxidant Abilities Are Associated with Lower Susceptibility of Hypertension in Middle-Aged Korean Participants: Impaired HDL Quality and Hypertension Risk
Abstract
1. Introduction
2. Results
2.1. Distribution of Age, Blood Pressure, and Body Mass Index
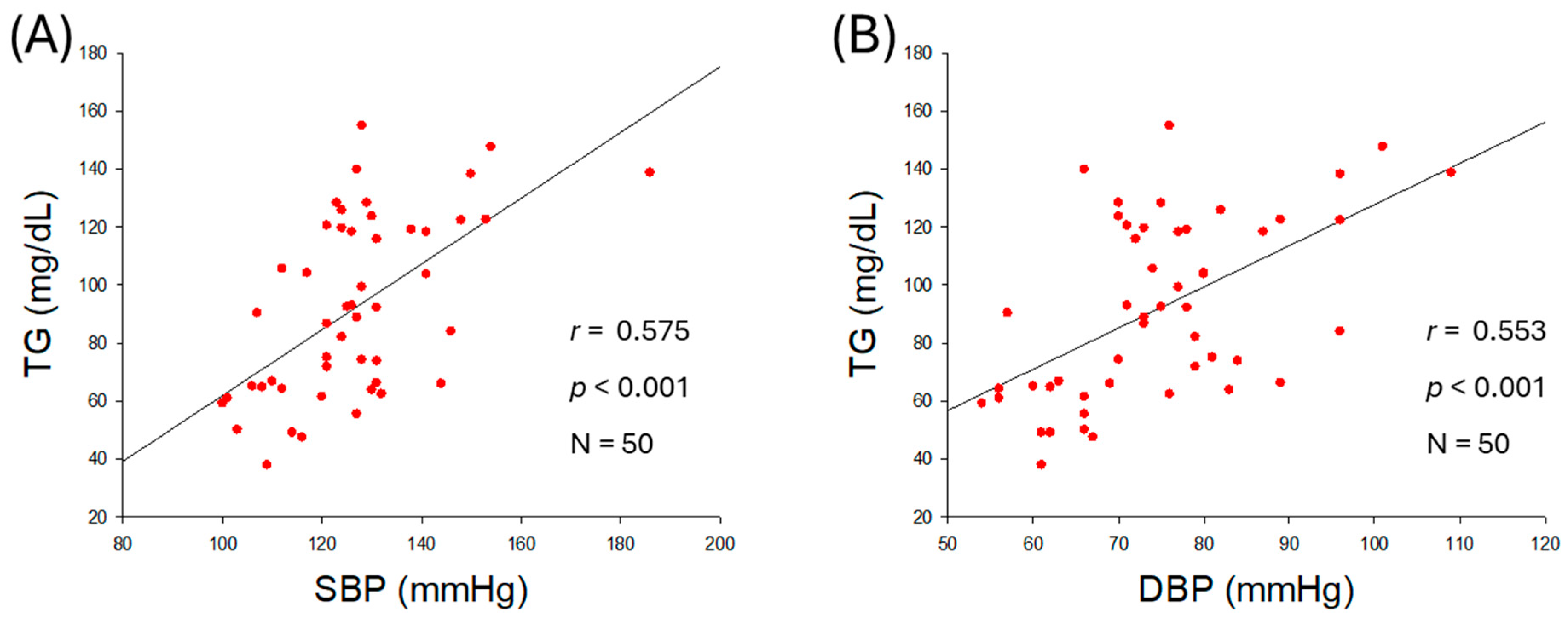
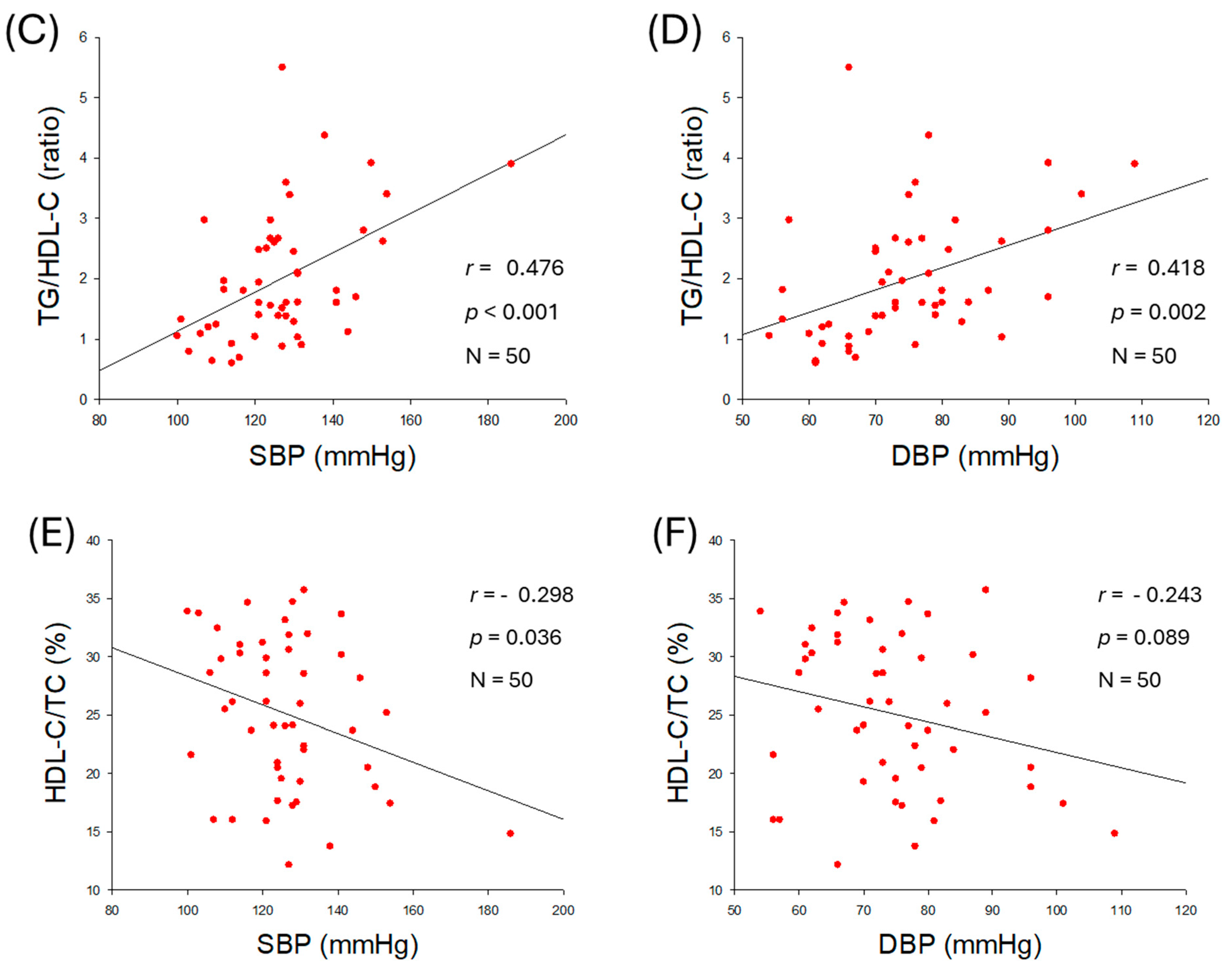
2.2. Correlation of Blood Lipid Profiles and Blood Pressure
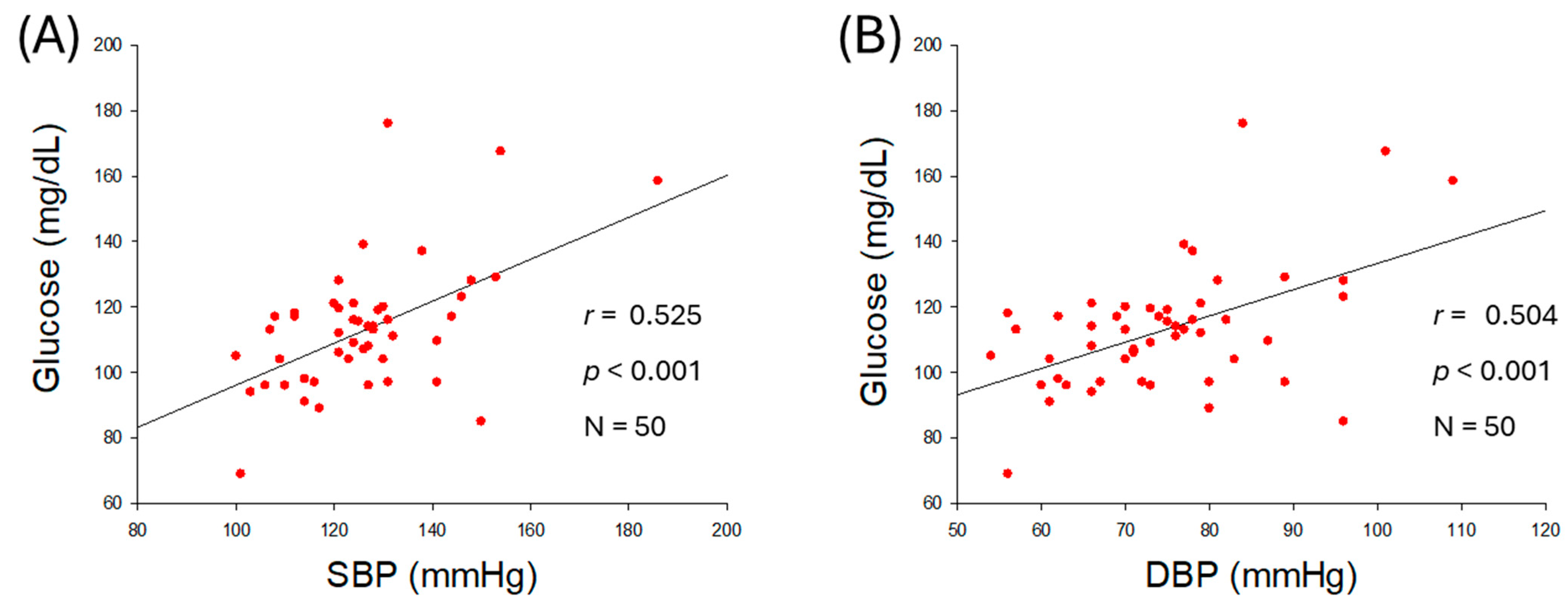
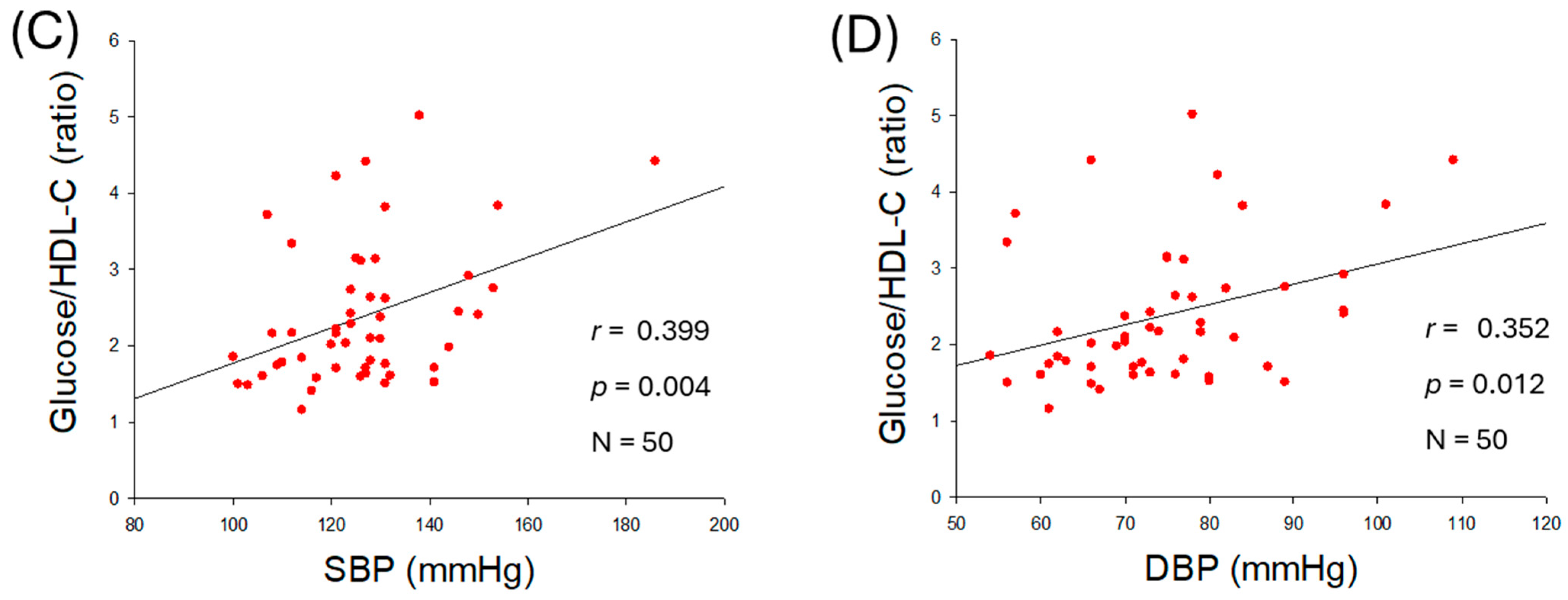
2.3. Correlation of Serum Glucose and Blood Pressure
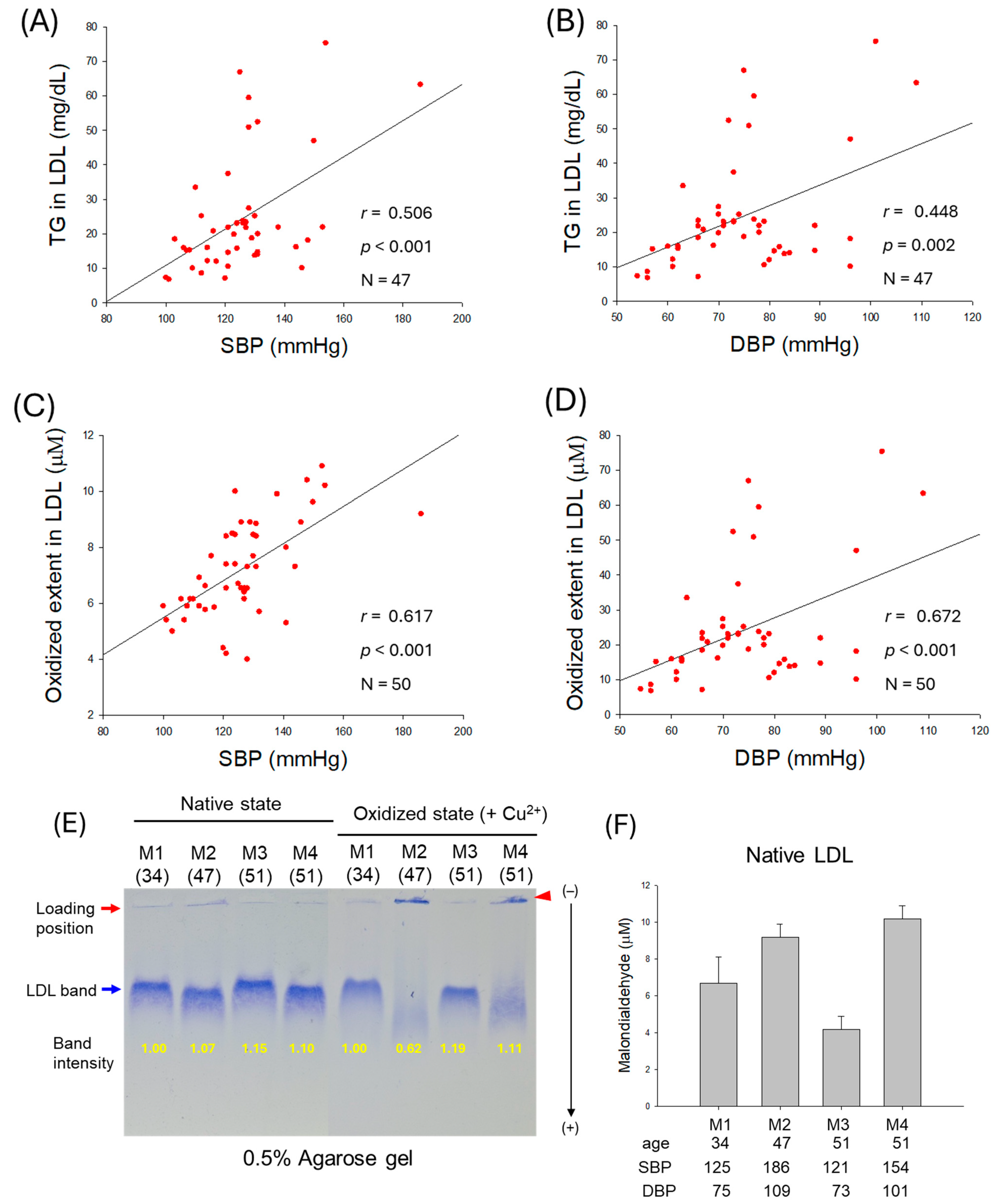
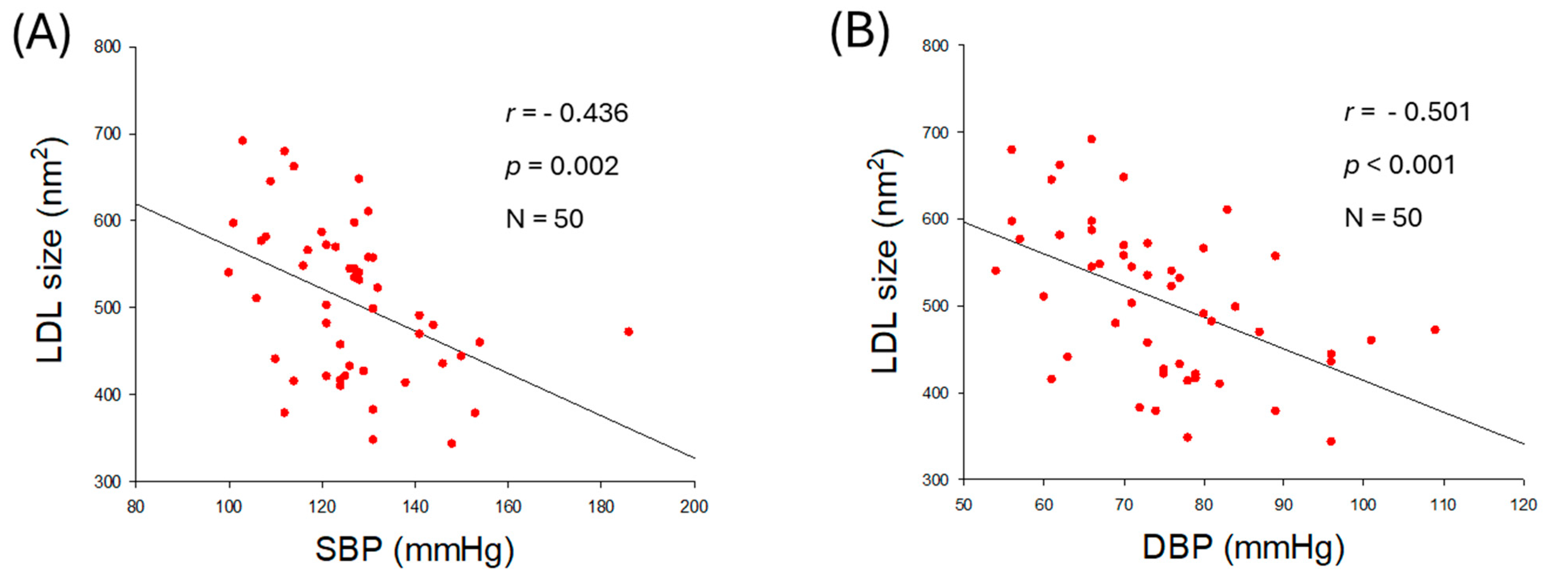
2.4. Correlation of LDL Quality and Blood Pressure
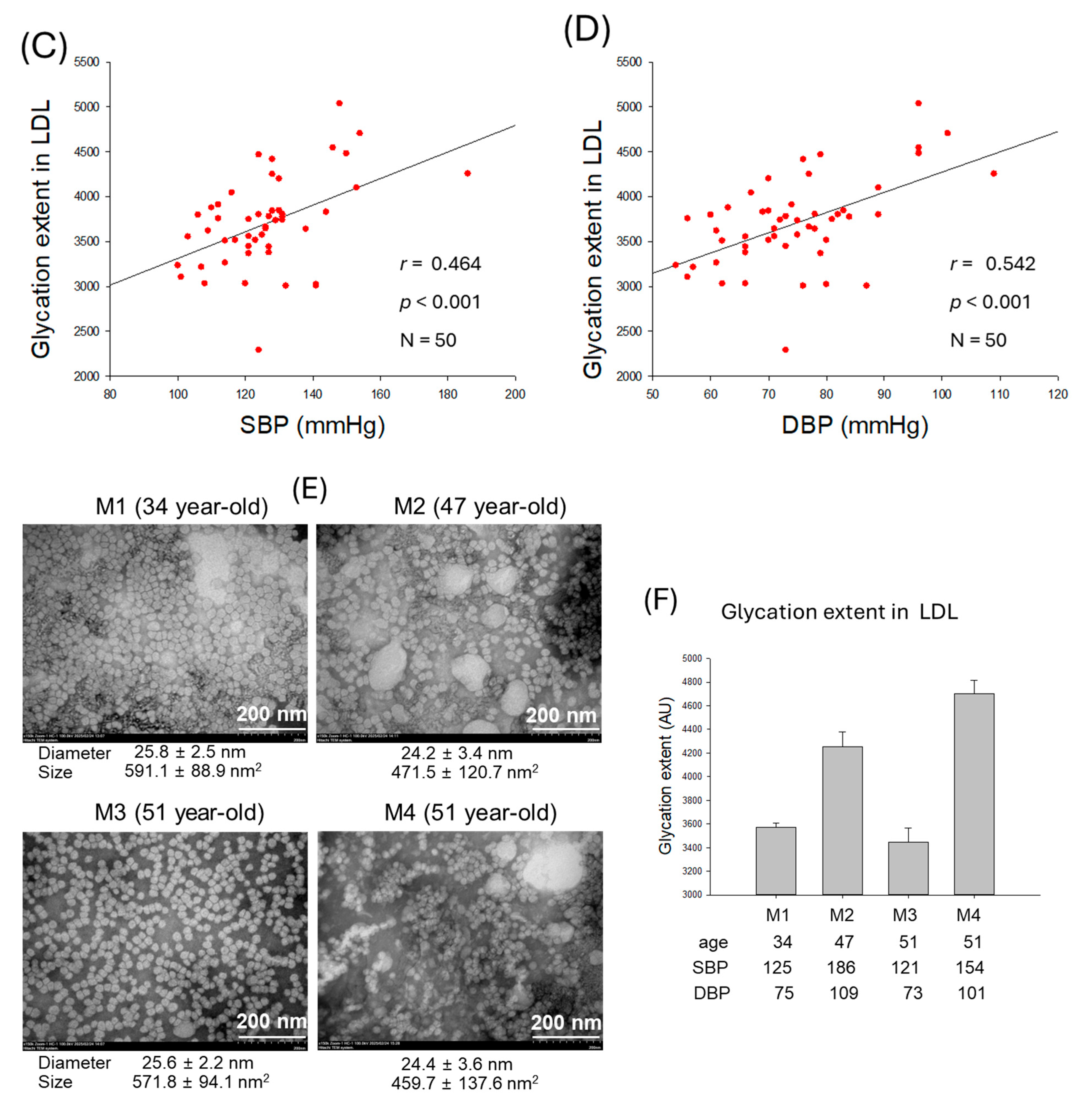
2.5. Particle Size and Glycation Extent of LDL
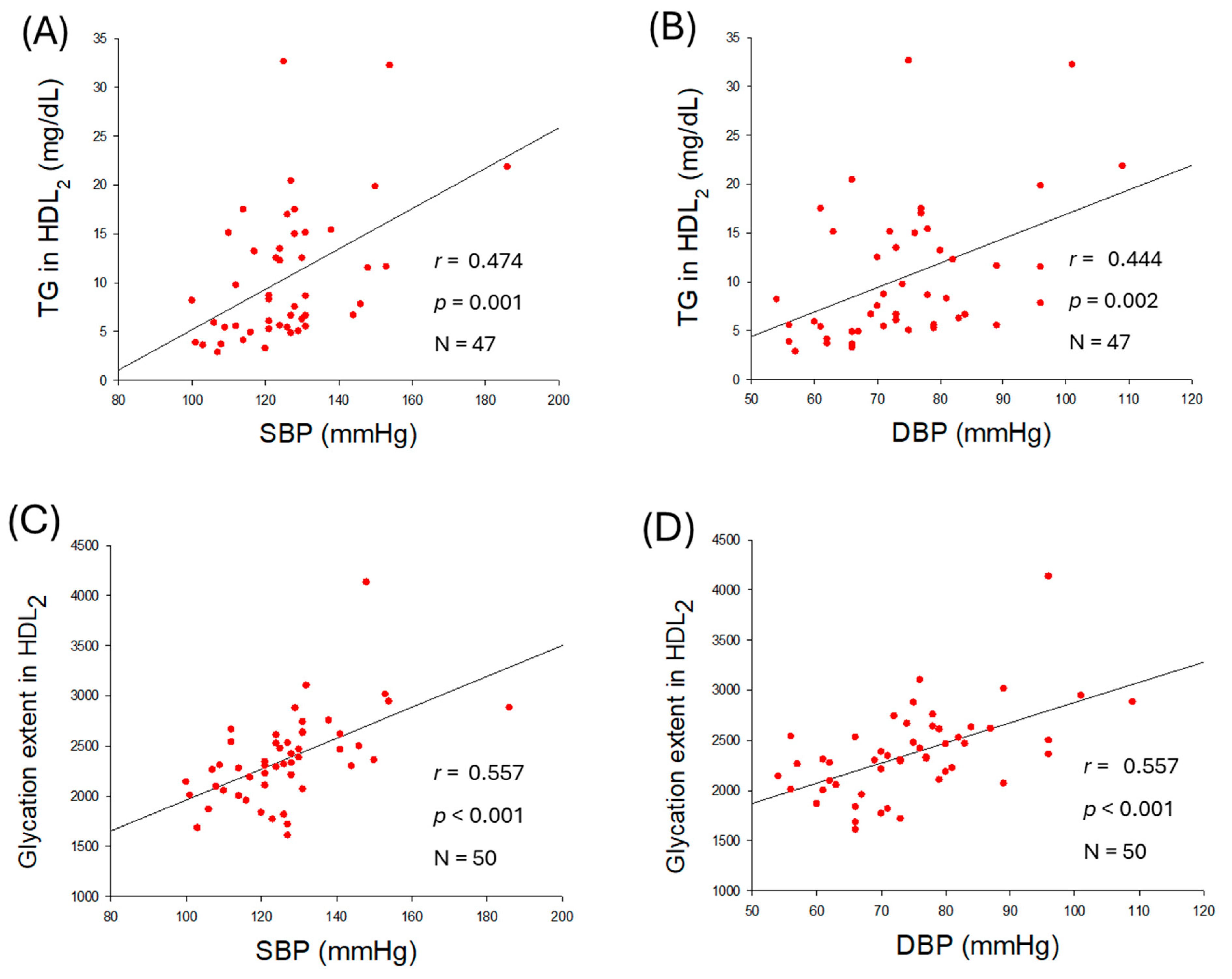
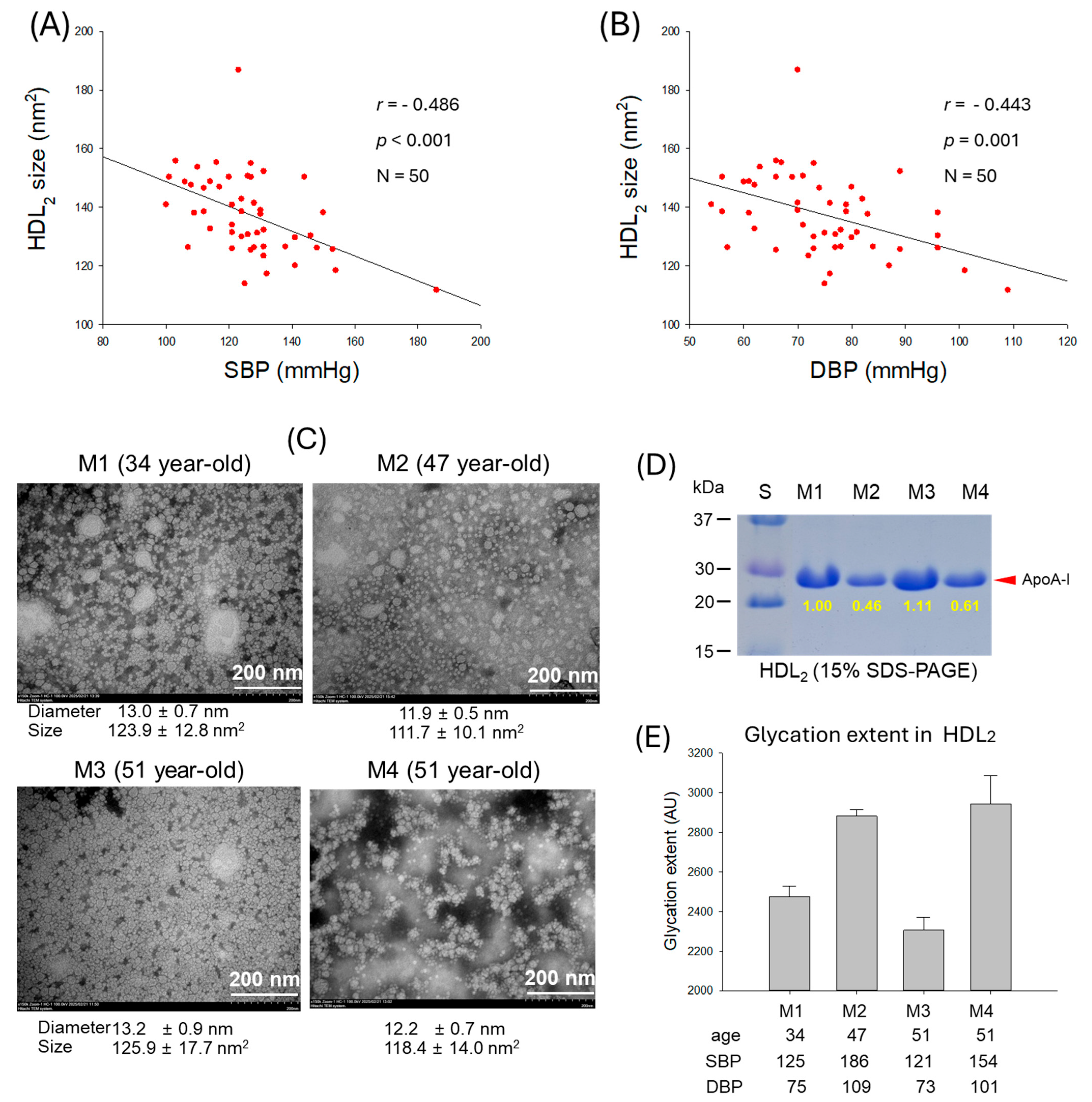
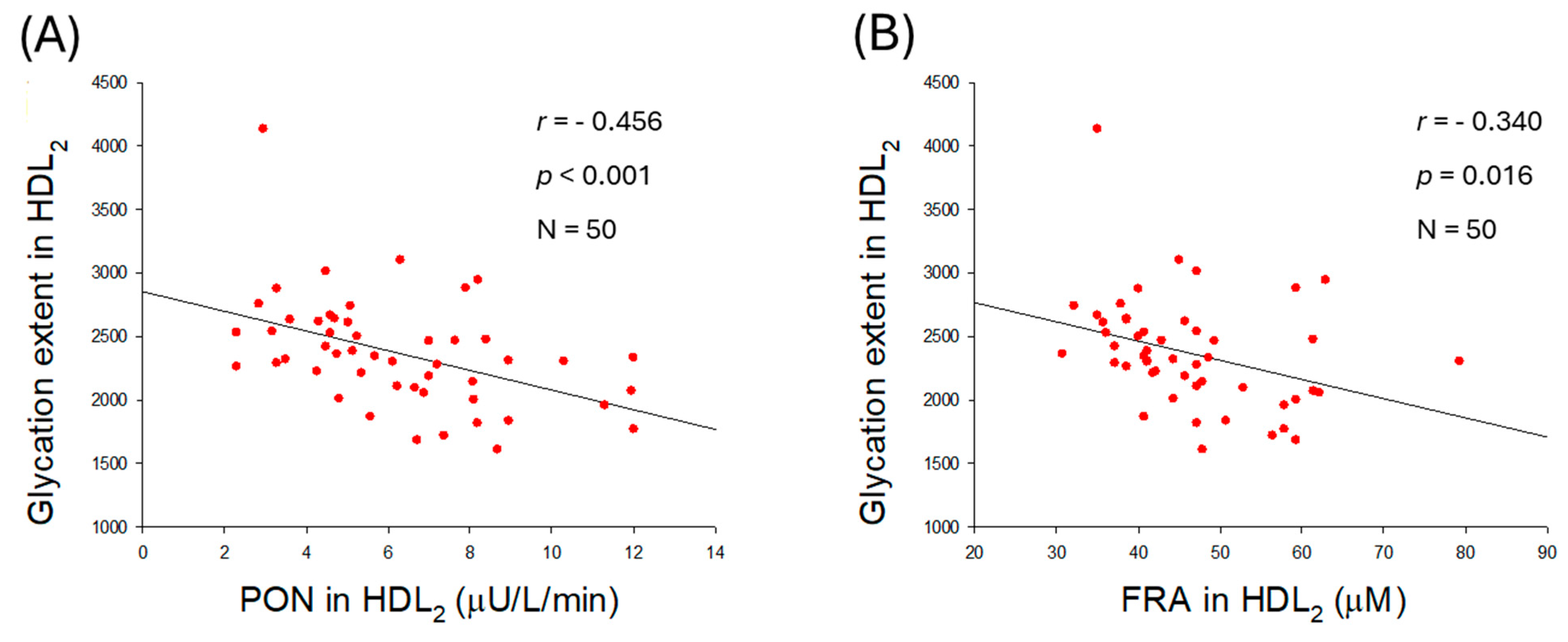
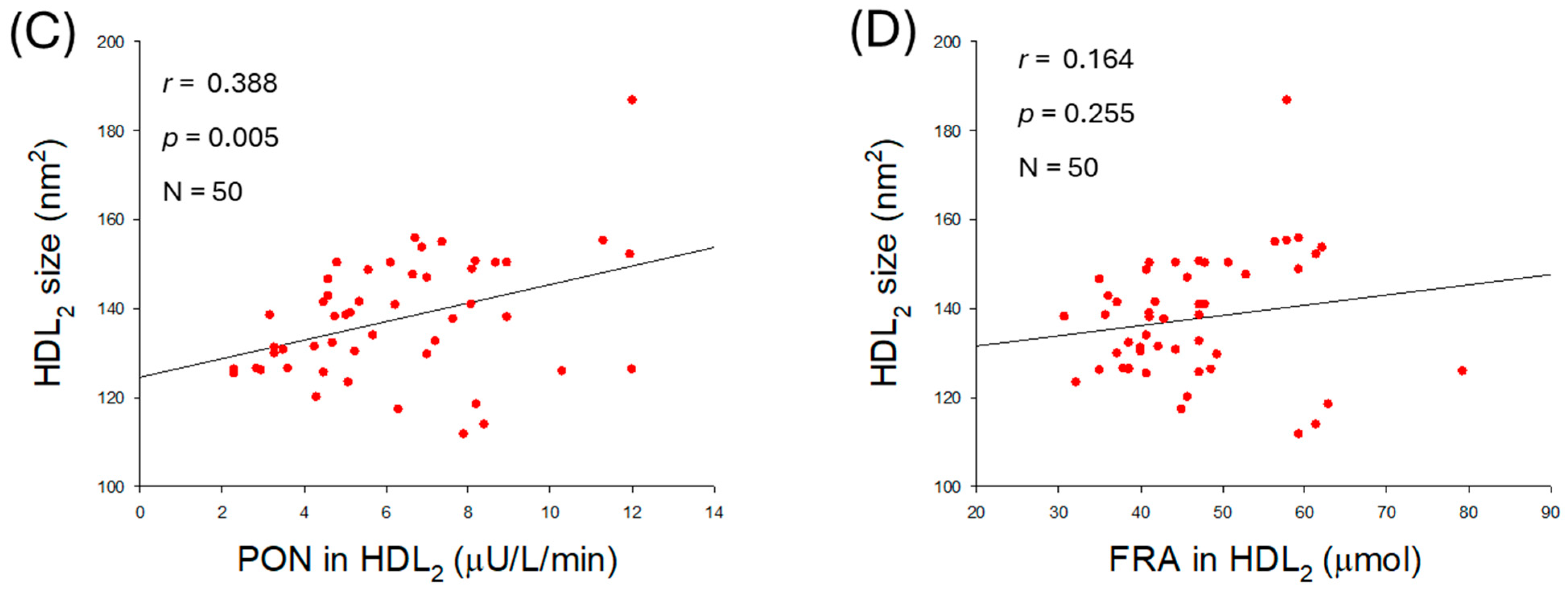
2.6. HDL2 Quality and Correlation with Blood Pressure
2.7. Correlation of HDL2 Size and Blood Pressure
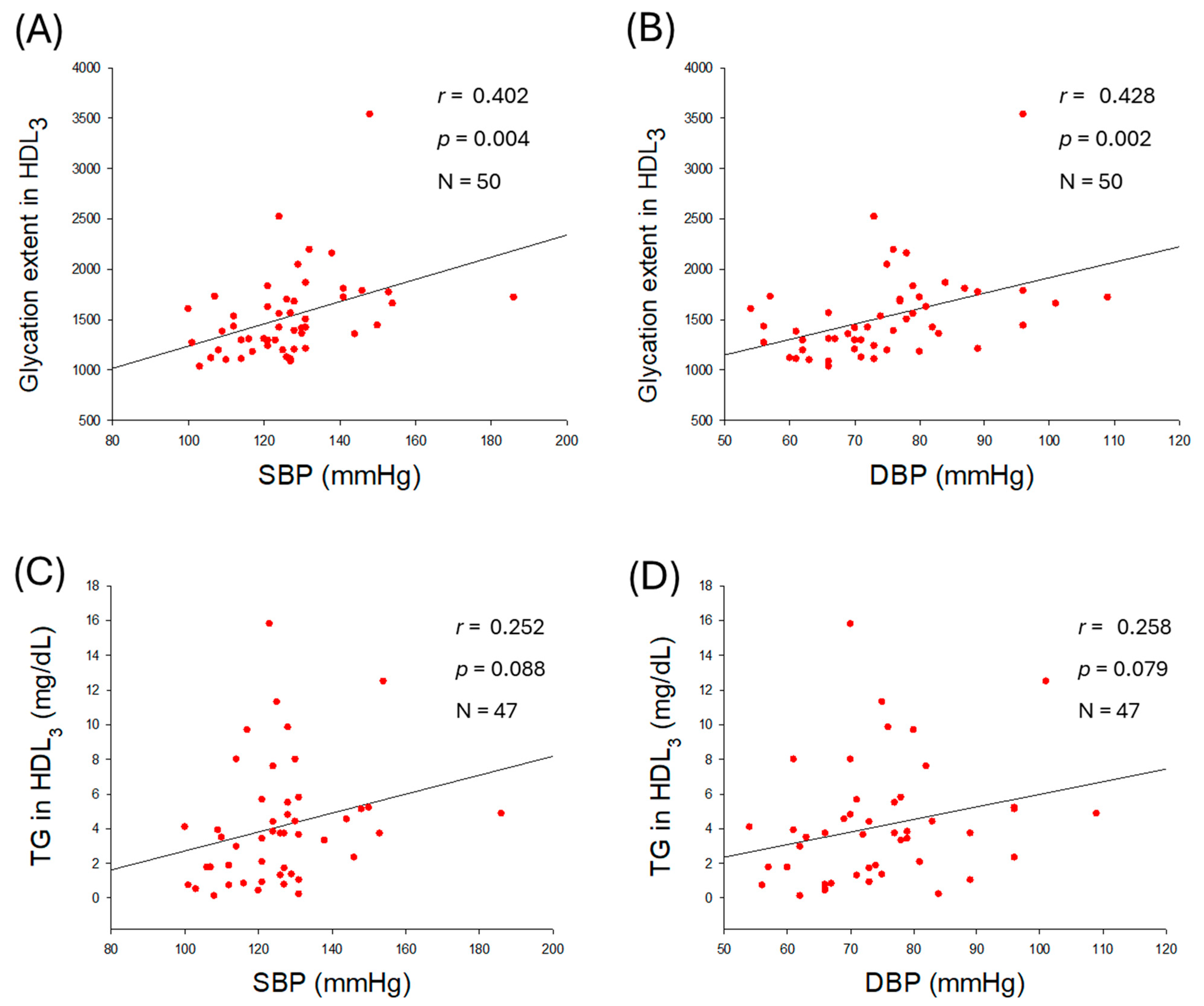
2.8. Glycation Extent in HDL3 and Correlation with Blood Pressure
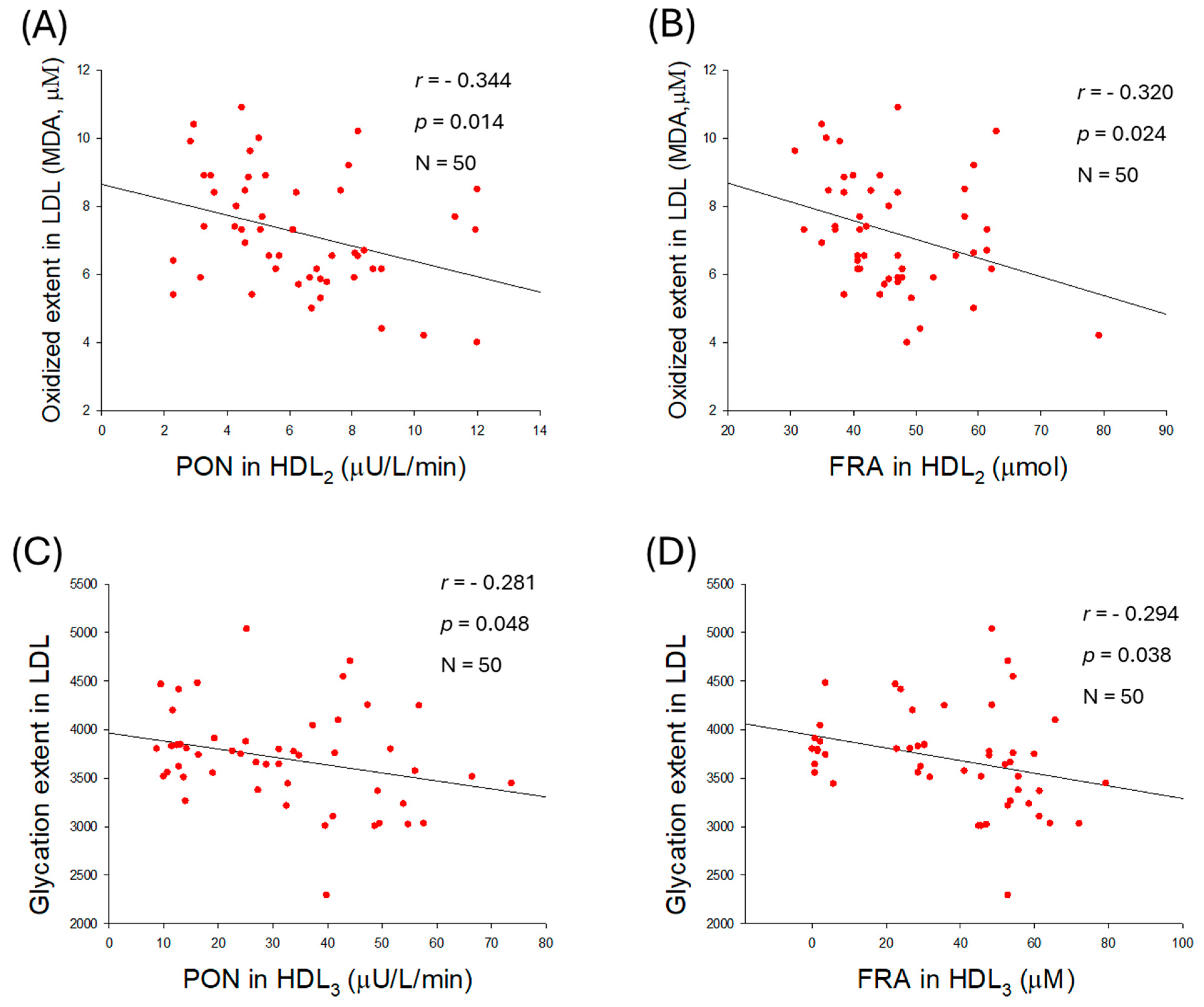
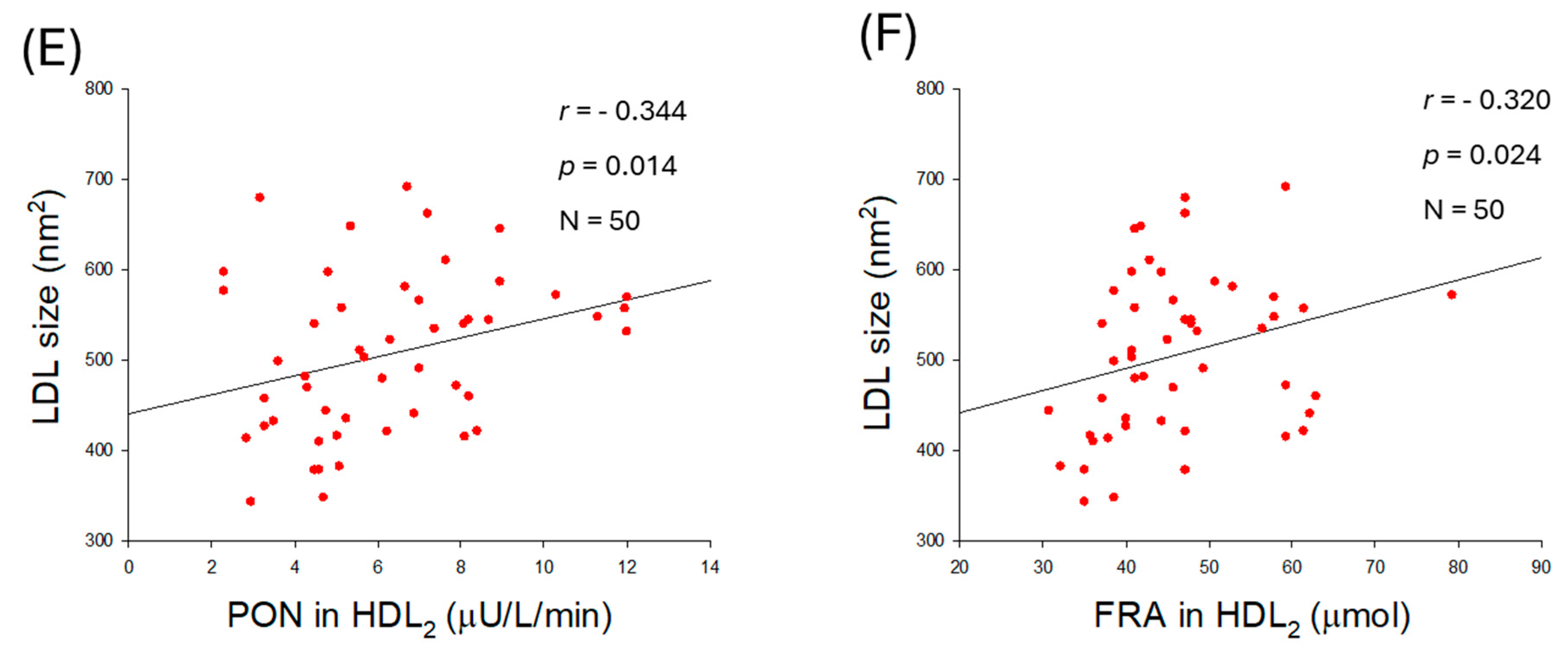
2.9. Correlation of LDL Qualities and Antioxidant Abilities in HDL2
2.10. Quality of HDL2 and Antioxidant Abilities of HDL2
2.11. Correlation Analysis of Parameters Between SBP and DBP
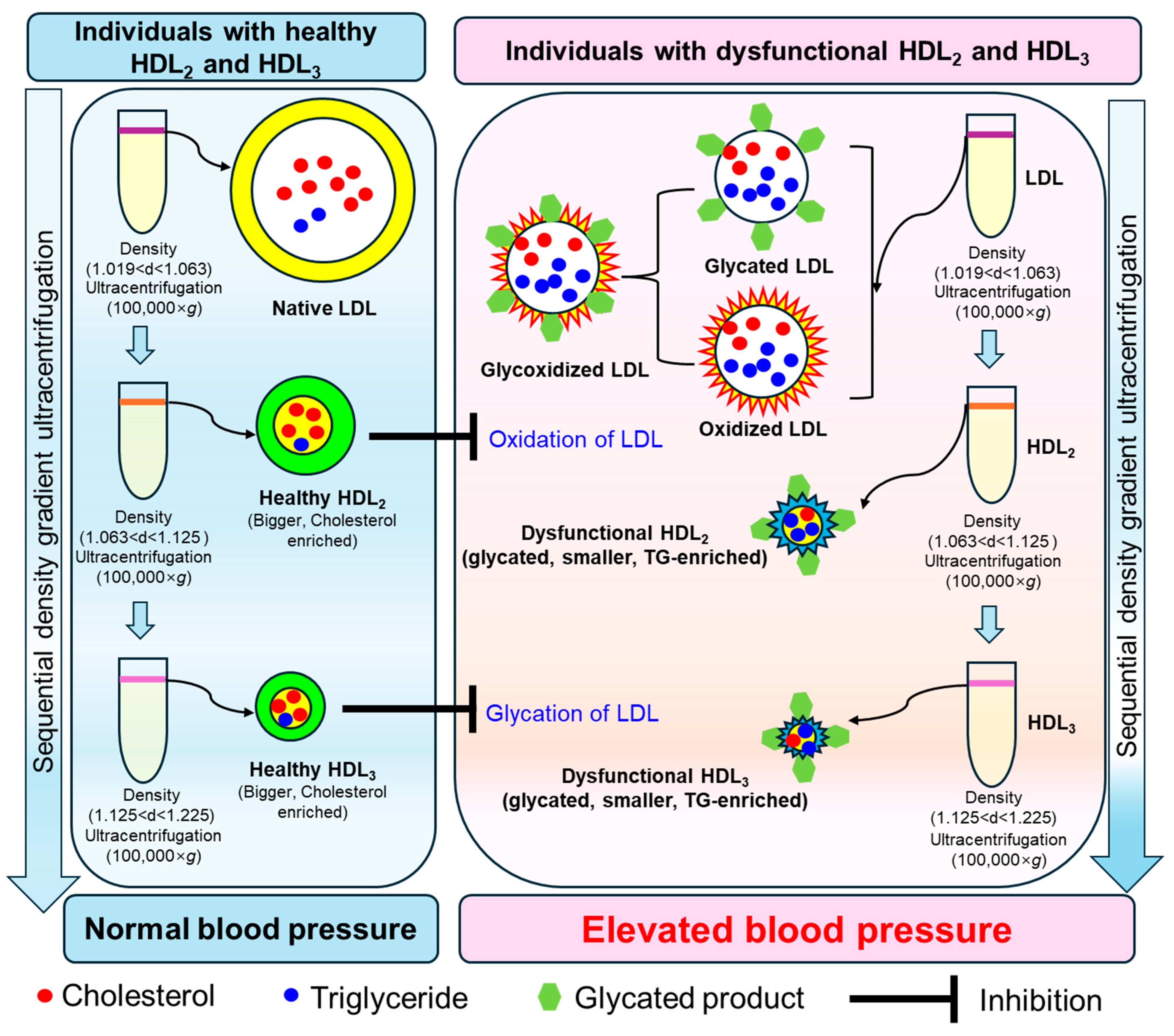
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Anthropometric Analysis
4.3. Blood Analysis
4.4. Isolation of Lipoproteins
4.5. Quantification of LDL Oxidation and Agarose Gel Electrophoresis
4.6. Paraoxonase Assay
4.7. Ferric Ion Reducing Ability Assay
4.8. Electrophoresis of HDL
4.9. Glycation Extent of LDL, HDL2, and HDL3
4.10. Electron Microscopy
4.11. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.P.; Zhao, S.P.; Zhang, X.Y.; Liu, L.; Gao, M.; Zhou, Q.C. Protective effect of high density lipoprotein on endothelium-dependent vasodilatation. Int. J. Cardiol. 2000, 73, 231–236. [Google Scholar] [CrossRef]
- Shen, Y.; Shi, L.; Nauman, E.; Katzmarzyk, P.T.; Price-Haywood, E.G.; Bazzano, A.N.; Nigam, S.; Hu, G. Inverse association between HDL (High-Density Lipoprotein) cholesterol and stroke risk among patients with type 2 diabetes mellitus. Stroke 2019, 50, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Taggart, H.; Stout, R.W. Reduced high density lipoprotein in stroke: Relationship with elevated triglyceride and hypertension. Eur. J. Clin. Investig. 1979, 9, 219–221. [Google Scholar] [CrossRef]
- Liu, X.; Tao, L.; Cao, K.; Wang, Z.; Chen, D.; Guo, J.; Zhu, H.; Yang, X.; Wang, Y.; Wang, J.; et al. Association of high-density lipoprotein with development of metabolic syndrome components: A five-year follow in adults. BMC Public Health 2015, 15, 412. [Google Scholar] [CrossRef]
- Yang, G.; Qian, T.; Sun, H.; Xu, Q.; Hou, X.; Hu, W.; Zhang, G.; Drummond, G.R.; Sobey, C.G.; Witting, P.K.; et al. Adjustment for body mass index changes inverse associations of HDL-cholesterol with blood pressure and hypertension to positive associations. J. Hum. Hypertens. 2022, 36, 570–579. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Kieneker, L.M.; Bakker, S.J.L.; James, R.W.; Dullaart, R.P.F. The inverse association of HDL-cholesterol with future risk of hypertension is not modified by its antioxidant constituent, paraoxonase-1: The PREVEND prospective cohort study. Atherosclerosis 2017, 263, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Von Eckardstein, A.; Nordestgaard, B.G.; Remaley, A.T.; Catapano, A.L. High-density lipoprotein revisited: Biological functions and clinical relevance. Eur. Heart J. 2022, 44, 1394–1407. [Google Scholar] [CrossRef]
- Ryu, H.E.; Jung, D.H.; Heo, S.J.; Park, B.; Lee, Y.J. Extremely high HDL cholesterol paradoxically increases the risk of all-cause mortality in non-diabetic males from the Korean population: Korean genome and epidemiology study-health examinees (KoGES-HEXA) cohorts. Front. Med. 2025, 12, 1534524. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Novel insights from human studies on the role of high-density lipoprotein in mortality and noncardiovascular disease. Arter. Thromb. Vasc. Biol. 2021, 41, 128–140. [Google Scholar] [CrossRef]
- Bonizzi, A.; Piuri, G.; Corsi, F.; Cazzola, R.; Mazzucchelli, S. HDL Dysfunctionality: Clinical Relevance of Quality Rather Than Quantity. Biomedicines 2021, 9, 729. [Google Scholar] [CrossRef]
- Bardagjy, A.S.; Steinberg, F.M. Relationship Between HDL Functional Characteristics and Cardiovascular Health and Potential Impact of Dietary Patterns: A Narrative Review. Nutrients 2019, 11, 1231. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dhindsa, D.; Almuwaqqat, Z.; Sun, Y.V.; Quyyumi, A.A. Very High High-Density Lipoprotein Cholesterol Levels and Cardiovascular Mortality. Am. J. Cardiol. 2022, 167, 43–53. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Martinez, I.; Sourlas, A.; Bouza, K.V.; Campos, F.N.; Torres, V.; Montan, P.D.; Guzman, E. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs Context 2018, 7, 212525. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H. The Current Status of Research on High-Density Lipoproteins (HDL): A Paradigm Shift from HDL Quantity to HDL Quality and HDL Functionality. Int. J. Mol. Sci. 2022, 23, 3967. [Google Scholar] [CrossRef]
- Rashid, S.; Watanabe, T.; Sakaue, T.; Lewis, G.F. Mechanisms of HDL lowering in insulin resistant, hypertriglyceridemic states: The combined effect of HDL triglyceride enrichment and elevated hepatic lipase activity. Clin. Biochem. 2003, 36, 421–429. [Google Scholar] [CrossRef]
- Smith, J.D. Dysfunctional HDL as a diagnostic and therapeutic target. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 151–155. [Google Scholar] [CrossRef]
- Feng, H.; Li, X.A. Dysfunctional high-density lipoprotein. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 156–162. [Google Scholar] [CrossRef]
- Sakata, N.; Uesugi, N.; Takebayashi, S.; Nagai, R.; Jono, T.; Horiuchi, S.; Takeya, M.; Itabe, H.; Takano, T.; Myint, T. Glycoxidation and lipid peroxidation of low-density lipoprotein can synergistically enhance atherogenesis. Cardiovasc. Res. 2001, 49, 466–475. [Google Scholar] [CrossRef]
- Younis, N.; Charlton-Menys, V.; Sharma, R.; Soran, H.; Durrington, P.N. Glycation of LDL in non-diabetic people: Small dense LDL is preferentially glycated both in vivo and in vitro. Atherosclerosis 2009, 202, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yang, S.; Lu, J.; Wu, M. Small, Dense Low-Density Lipoprotein-Cholesterol and Atherosclerosis: Relationship and Therapeutic Strategies. Front. Cardiovasc. Med. 2021, 8, 804214. [Google Scholar] [CrossRef]
- Hidaka, A.; Inoue, K.; Kutsukake, S.; Adachi, M.; Kakuta, Y.; Kojo, S. Decrease in the particle size of low-density lipoprotein (LDL) by oxidation. Bioorg. Med. Chem. Lett. 2005, 15, 2781–2785. [Google Scholar] [CrossRef]
- Bentley, A.R.; Rotimi, C.N. Interethnic variation in lipid profiles: Implications for underidentification of African–Americans at risk for metabolic disorders. Exp. Rev. Endocrinol. Metab. 2012, 7, 659–667. [Google Scholar] [CrossRef]
- Zakai, N.A.; Minnier, J.; Safford, M.M.; Koh, I.; Irvin, M.R.; Fazio, S.; Cushman, M.; Howard, V.J.; Pamir, N. Race-dependent association of high-density lipoprotein cholesterol levels with incident coronary artery disease. J. Am. Coll. Cardiol. 2022, 80, 2104–2115. [Google Scholar] [CrossRef]
- Cho, K.-H.; Park, H.-J.; Kim, J.-R. Decrease in serum HDL-C level is associated with elevation of blood pressure: Correlation analysis from the Korean National Health and nutrition examination survey 2017. Int. J. Environ. Res. Public Health 2020, 17, 1101. [Google Scholar] [CrossRef]
- Cho, K.-H.; Park, H.-J.; Kim, S.-J.; Kim, J.-R. Decrease in HDL-C is associated with age and household income in adults from the Korean National Health and Nutrition Examination Survey 2017: Correlation analysis of low HDL-C and poverty. Int. J. Environ. Res. Public Health 2019, 16, 3329. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Picca, A.; Tosato, M.; Martone, A.M.; Ortolani, E.; Sisto, A.; D’Angelo, E.; Serafini, E.; Desideri, G.; et al. Body mass index is strongly associated with hypertension: Results from the longevity check-up 7+ study. Nutrients 2018, 10, 1976. [Google Scholar] [CrossRef] [PubMed]
- Goswami, B.; Reang, T.; Sarkar, S.; Sengupta, S.; Bhattacharjee, B. Role of body visceral fat in hypertension and dyslipidemia among the diabetic and nondiabetic ethnic population of Tripura—A comparative study. J. Fam. Med. Prim. Care 2020, 9, 2885. [Google Scholar] [CrossRef]
- Deng, G.; Li, Y.; Cheng, W. Association of lipid levels with the prevalence of hypertension in Chinese women: A cross-sectional study based on 32 health Check centers. Front. Endocrinol. 2022, 13, 904237. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, X.; Lai, W.; Liu, J.; Zhou, B. Association between TG/HDL-C and hypertension in Chinese middle-aged and older adults: Findings from CHARLS. BMC Cardiovasc. Disord. 2025, 25, 254. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Virella, M.F.; Hunt, K.J.; Baker, N.L.; Lachin, J.; Nathan, D.M.; Virella, G. Levels of oxidized LDL and advanced glycation end products-modified LDL in circulating immune complexes are strongly associated with increased levels of carotid intima-media thickness and its progression in type 1 diabetes. Diabetes 2011, 60, 582–589. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and its impact on cardiovascular health: Focus on atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef] [PubMed]
- Toshima, S.; Hasegawa, A.; Kurabayashi, M.; Itabe, H.; Takano, T.; Sugano, J.; Shimamura, K.; Kimura, J.; Michishita, I.; Suzuki, T.; et al. Circulating oxidized low density lipoprotein levels. A biochemical risk marker for coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2243–2247. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Bowden, R.G. Assessing high-density lipoprotein: Shifting focus from quantity to quality in cardiovascular disease risk assessment. Int. J. Transl. Med. 2024, 4, 369–380. [Google Scholar] [CrossRef]
- Lamarche, B.; Uffelman, K.D.; Carpentier, A.; Cohn, J.S.; Steiner, G.; Barrett, P.H.; Lewis, G.F. Triglyceride enrichment of HDL enhances in vivo metabolic clearance of HDL apo A-I in healthy men. J. Clin. Investig. 1999, 103, 1191–1199. [Google Scholar] [CrossRef]
- Barter, P.J. The causes and consequences of low levels of high-density lipoproteins in patients with diabetes. Diabetes Metab. J. 2011, 35, 101–106. [Google Scholar] [CrossRef]
- Tian, L.; Xu, Y.; Fu, M.; Peng, T.; Liu, Y.; Long, S. The impact of plasma triglyceride and apolipoproteins concentrations on high-density lipoprotein subclasses distribution. Lipids Health Dis. 2011, 10, 17. [Google Scholar] [CrossRef]
- Amigó, N.; Torné, P.; Nordestgaard, L.T.; Di Giacomo-Barbagallo, F.; Merino, C.; Magni, P.; González-Lleó, A.; Andreychuk, N.; Catapano, A.L.; Masana, L.; et al. Triglycerides as Determinants of Global Lipoprotein Derangement: Implications for Cardiovascular Prevention. Int. J. Mol. Sci. 2025, 26, 8284. [Google Scholar] [CrossRef]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Sakuma, N.; Hibino, T.; Sato, T.; Fujinami, T. HDL3 exerts more powerful anti-oxidative, protective effects against copper-catalyzed LDL oxidation than HDL2. Clin. Biochem. 1997, 30, 221–225. [Google Scholar] [CrossRef]
- Huang, J.M.; Huang, Z.X.; Zhu, W. Mechanism of high-density lipoprotein subfractions inhibiting copper-catalyzed oxidation of low-density lipoprotein. Clin. Biochem. 1998, 31, 537–543. [Google Scholar] [CrossRef]
- Bacchetti, T.; Masciangelo, S.; Armeni, T.; Bicchiega, V.; Ferretti, G. Glycation of human high density lipoprotein by methylglyoxal: Effect on HDL-paraoxonase activity. Metabolism 2014, 63, 307–311. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects; World Medical Association: Helsinki, Finland, 2024. [Google Scholar]
- National High Blood Pressure Education Program. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. 2004. Available online: https://www.nhlbi.nih.gov/files/docs/guidelines/jnc7full.pdf (accessed on 11 January 2026).
- Cho, K.H.; Nam, H.S.; Baek, S.H.; Kang, D.J.; Na, H.; Komatsu, T.; Uehara, Y. Beneficial effect of Cuban policosanol on blood pressure and serum lipoproteins accompanied with lowered glycated hemoglobin and enhanced high-density lipoprotein functionalities in a randomized, placebo-controlled, and double-blinded trial with healthy Japanese. Int. J. Mol. Sci. 2023, 24, 5185. [Google Scholar]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef]
- Markwell, M.A.C.; Haas, S.M.; Biebar, L.L.; Tolbert, N.E. A modification of the Lowry procedure to simplify protein determination in membrane and in protein samples. Anal. Biochem 1978, 87, 206–211. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Morgan, J.; Leake, D. Oxidation of low density lipoprotein by iron or copper at acidic pH. J. Lipid Res. 1995, 36, 2504–2512. [Google Scholar] [CrossRef]
- Noble, R.P. Electrophoretic separation of plasma lipoproteins in agarose gel. J. Lipid Res. 1968, 9, 693–700. [Google Scholar] [CrossRef]
- Blatter Garin, M.-C.; Moren, X.; James, R.W. Paraoxonase-1 and serum concentrations of HDL-cholesterol and apoA-I. J. Lipid Res. 2006, 47, 515–520. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [PubMed]
- McPherson, J.D.; Shilton, B.H.; Walton, D.J. Role of fructose in glycation and cross-linking of proteins. Biochemistry 1988, 27, 1901–1907. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-R.; Lee, I.-C.; Kwon, H.-J. Native high-density lipoproteins (HDL) with higher paraoxonase exerts a potent antiviral effect against SARS-CoV-2 (COVID-19), while glycated HDL lost the antiviral activity. Antioxidants 2021, 10, 209. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 50) | Male (n = 25) | Female (n = 25) | p | ||
|---|---|---|---|---|---|
| Age | 47.0 ± 11.7 | 49.2 ± 11.7 | 44.8 ± 11.5 | 0.195 | |
| SBP (mmHg) | 126.3 ± 15.8 | 132.7 ± 14.6 | 119.9 ± 14.4 | 0.003 | |
| DBP (mmHg) | 74.8 ± 12.1 | 79.8 ± 10.8 | 69.8 ± 11.5 | 0.003 | |
| BMI | 24.6 ± 4.5 | 26.7 ± 4.6 | 22.6 ± 3.3 | 0.003 | |
| Body fat (%) | 26.3 ± 4.8 | 24.7 ± 4.1 | 27.9 ± 5.1 | 0.019 | |
| Subcutaneous fat (kg) | 15.5 ± 4.4 | 16.0 ± 4.2 | 15.0 ± 4.7 | 0.411 | |
| Visceral fat (kg) | 2.4 ± 1.1 | 2.9 ± 1.0 | 1.8 ± 1.0 | 0.001 | |
| Serum | TC (mg/dL) | 208.1 ± 25.1 | 209.5 ± 20.9 | 206.7 ± 29.1 | 0.844 |
| TG (mg/dL) | 92.4 ± 31.0 | 107.2 ± 26.8 | 77.7 ± 28.9 | 0.001 | |
| HDL-C (mg/dL) | 51.3 ± 11.8 | 48.6 ± 12.2 | 53.9 ± 11.0 | 0.123 | |
| LDL-C (mg/dL) | 137.3 ± 25.1 | 139.5 ± 23.0 | 135.1 ± 27.3 | 0.669 | |
| TG/HDL-C (ratio) | 2.0 ± 1.1 | 2.4 ± 1.1 | 1.6 ± 0.9 | 0.004 | |
| LDL/HDL-C (ratio) | 2.9 ± 1.1 | 3.2 ± 1.3 | 2.7 ± 0.9 | 0.181 | |
| HDL-C/TC (%) | 25.1 ± 6.5 | 23.7 ± 6.7 | 26.5 ± 6.1 | 0.138 | |
| Glucose (mg/dL) | 113.3 ± 18.9 | 122.0 ± 20.1 | 104.6 ± 4.1 | 0.001 | |
| Glucose/HDL-C (ratio) | 2.4 ± 0.9 | 2.7 ± 1.0 | 2.0 ± 0.6 | 0.009 | |
| LDL | TC (mg/dL) | 126.9 ± 38.4 | 123.2 ± 45.0 | 130.2 ± 32.2 | 0.540 |
| TG (mg/dL) | 24.3 ± 16.5 | 32.1 ± 19.6 | 17.4 ± 8.9 | 0.003 | |
| Oxidized extent (µM) | 7.2 ± 1.7 | 7.5 ± 1.7 | 7.0 ± 1.6 | 0.261 | |
| Size (nm2) | 506.5 ± 88.1 | 478.4 ± 89.9 | 534.6 ± 89.3 | 0.022 | |
| Diameter (nm) | 25.2 ± 2.3 | 24.4 ± 2.1 | 25.9 ± 2.2 | 0.022 | |
| Glycation extent | 3702 ± 502 | 3737 ± 591 | 3667 ± 404 | 0.628 | |
| HDL2 | TC (mg/dL) | 51.3 ± 17.0 | 47.2 ± 17.8 | 54.8 ± 15.8 | 0.127 |
| TG (mg/dL) | 10.5 ± 6.9 | 13.7 ± 7.7 | 7.6 ± 4.7 | 0.002 | |
| PON (µU/L/min) | 6.3 ± 2.5 | 6.0 ± 2.8 | 6.5 ± 2.3 | 0.460 | |
| FRA (μM) | 46.4 ± 9.8 | 45.6 ± 11.1 | 47.2 ± 8.5 | 0.587 | |
| Size (nm2) | 137.6 ± 13.7 | 132.2 ± 15.5 | 143.0 ± 9.1 | 0.004 | |
| Diameter (nm) | 13.2 ± 0.6 | 12.9 ± 0.7 | 13.4 ± 0.4 | 0.002 | |
| Glycation extent | 2367 ± 435 | 2516 ± 486 | 2218 ± 323 | 0.014 | |
| HDL3 | TC (mg/dL) | 21.0 ± 5.5 | 21.2 ± 7.0 | 20.8 ± 3.8 | 0.801 |
| TG (mg/dL) | 4.1 ± 3.4 | 5.1 ± 4.1 | 3.3 ± 2.6 | 0.075 | |
| PON (µU/L/min) | 31.9 ± 17.1 | 35.4 ± 17.6 | 28.5 ± 16.3 | 0.156 | |
| FRA (μM) | 36.8 ± 22.6 | 39.6 ± 20.5 | 33.9 ± 24.7 | 0.377 | |
| Glycation extent | 1527 ± 432 | 1677 ± 519 | 1377 ± 255 | 0.013 | |
| SBP (mmHg) | DBP (mmHg) | ||||
|---|---|---|---|---|---|
| r | p | r | p | ||
| Body | Age | 0.245 | 0.087 | 0.132 | 0.360 |
| BMI | 0.362 | 0.011 | 0.360 | 0.011 | |
| Body fat (%) | 0.135 | 0.354 | 0.125 | 0.393 | |
| Subcutaneous fat (kg) | 0.248 | 0.386 | 0.235 | 0.104 | |
| Visceral fat (kg) | 0.407 | 0.004 | 0.381 | 0.007 | |
| Serum | TC (mg/dL) | 0.222 | 0.121 | 0.117 | 0.419 |
| TG (mg/dL) | 0.575 | <0.001 | 0.553 | <0.001 | |
| HDL-C (mg/dL) | −0.238 | 0.096 | −0.234 | 0.102 | |
| LDL-C (mg/dL) | 0.226 | 0.115 | 0.139 | 0.336 | |
| TG/HDL-C (ratio) | 0.476 | <0.001 | 0.418 | 0.002 | |
| LDL/HDL-C (ratio) | 0.242 | 0.091 | 0.157 | 0.275 | |
| HDL-C/TC (%) | −0.298 | 0.036 | −0.243 | 0.089 | |
| Glucose (mg/dL) | 0.525 | <0.001 | 0.504 | <0.001 | |
| Glucose/HDL-C (ratio) | 0.399 | 0.004 | 0.352 | 0.012 | |
| LDL | TC (mg/dL) | 0.063 | 0.672 | 0.056 | 0.710 |
| TG (mg/dL) | 0.506 | <0.001 | 0.448 | 0.002 | |
| Oxidized extent (MDA) | 0.617 | <0.001 | 0.672 | <0.001 | |
| Size (nm2) | −0.436 | 0.002 | −0.501 | <0.001 | |
| Diameter (nm) | −0.447 | <0.001 | −0.501 | <0.001 | |
| Glycation extent | 0.464 | <0.001 | 0.542 | <0.001 | |
| HDL2 | TC (mg/dL) | −0.069 | 0.644 | −0.066 | 0.660 |
| TG (mg/dL) | 0.474 | <0.001 | 0.444 | 0.002 | |
| PON (µU/L/min) | −0.073 | 0.613 | −0.047 | 0.746 | |
| FRA (µM) | −0.050 | 0.733 | −0.044 | 0.762 | |
| Size (nm2) | −0.486 | <0.001 | −0.443 | 0.001 | |
| Diameter (nm) | −0.500 | <0.001 | −0.422 | 0.002 | |
| Glycation extent | 0.557 | <0.001 | 0.557 | <0.001 | |
| HDL3 | TC (mg/dL) | 0.035 | <0.001 | −0.007 | 0.961 |
| TG (mg/dL) | 0.252 | 0.088 | 0.258 | 0.079 | |
| PON (µU/L/min) | 0.078 | 0.590 | 0.061 | 0.675 | |
| FRA (µM) | 0.088 | 0.545 | 0.048 | 0.740 | |
| Glycation extent | 0.402 | 0.004 | 0.428 | 0.002 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Cho, K.-H.; Yang, C.-E.; Lee, S.H.; Lee, Y.; Bahuguna, A. Enhanced Qualities of High-Density Lipoproteins (HDLs) with Antioxidant Abilities Are Associated with Lower Susceptibility of Hypertension in Middle-Aged Korean Participants: Impaired HDL Quality and Hypertension Risk. Int. J. Mol. Sci. 2026, 27, 1108. https://doi.org/10.3390/ijms27021108
Cho K-H, Yang C-E, Lee SH, Lee Y, Bahuguna A. Enhanced Qualities of High-Density Lipoproteins (HDLs) with Antioxidant Abilities Are Associated with Lower Susceptibility of Hypertension in Middle-Aged Korean Participants: Impaired HDL Quality and Hypertension Risk. International Journal of Molecular Sciences. 2026; 27(2):1108. https://doi.org/10.3390/ijms27021108
Chicago/Turabian StyleCho, Kyung-Hyun, Chae-Eun Yang, Sang Hyuk Lee, Yunki Lee, and Ashutosh Bahuguna. 2026. "Enhanced Qualities of High-Density Lipoproteins (HDLs) with Antioxidant Abilities Are Associated with Lower Susceptibility of Hypertension in Middle-Aged Korean Participants: Impaired HDL Quality and Hypertension Risk" International Journal of Molecular Sciences 27, no. 2: 1108. https://doi.org/10.3390/ijms27021108
APA StyleCho, K.-H., Yang, C.-E., Lee, S. H., Lee, Y., & Bahuguna, A. (2026). Enhanced Qualities of High-Density Lipoproteins (HDLs) with Antioxidant Abilities Are Associated with Lower Susceptibility of Hypertension in Middle-Aged Korean Participants: Impaired HDL Quality and Hypertension Risk. International Journal of Molecular Sciences, 27(2), 1108. https://doi.org/10.3390/ijms27021108







