Systemic Treatment Strategies for Patients with Psoriasis and Psoriatic Arthritis in the Setting of ANA Positivity or Lupus Spectrum Disease: A Comprehensive Systematic Review
Abstract
1. Introduction
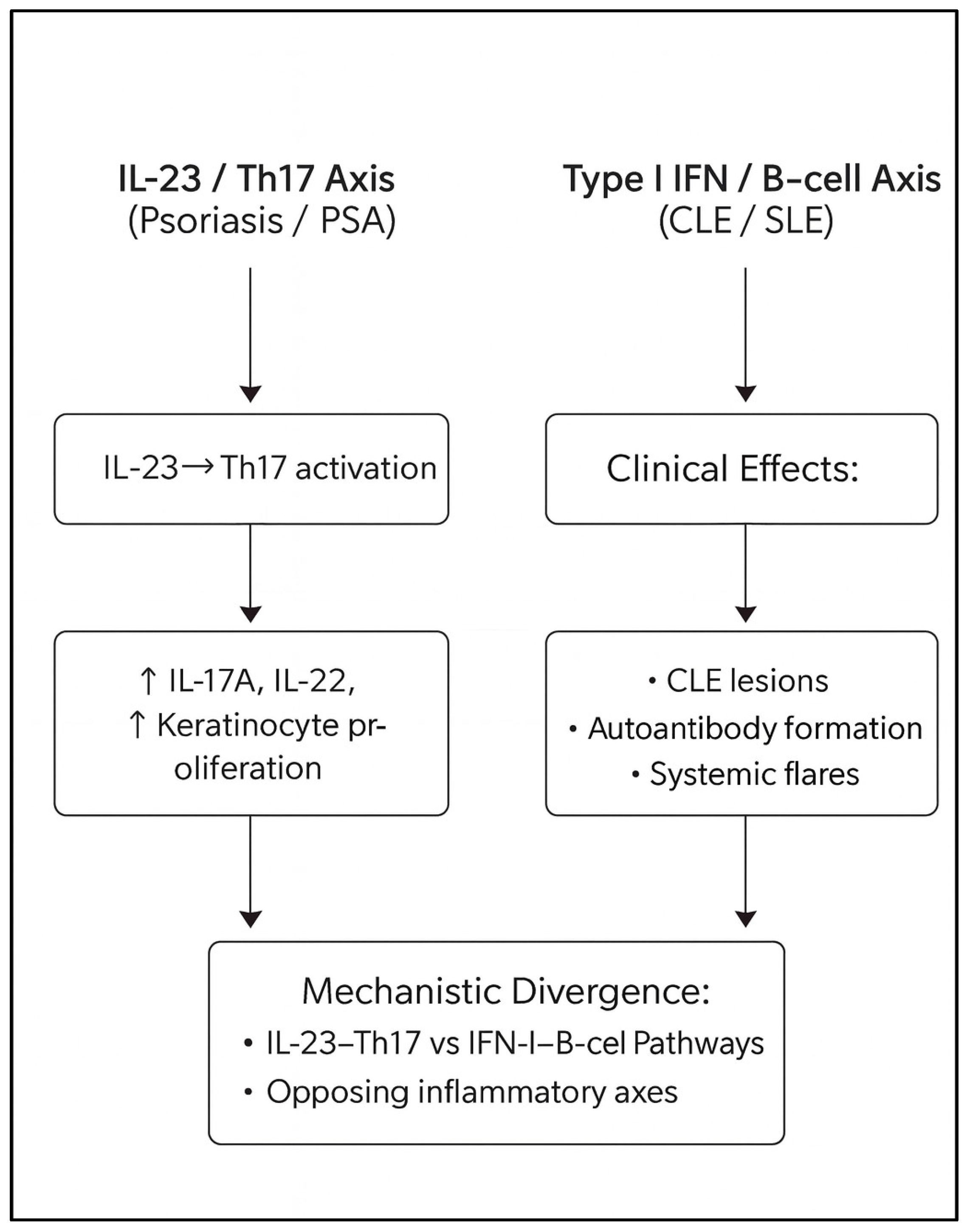
1.1. Background and Immunologic Divergence
1.2. Clinical Dilemma and Objective of This Review
2. Materials and Methods
2.1. Study Design and Reporting Standards
2.2. Eligibility Criteria
2.2.1. Study Eligibility Criteria
- Population.
- Interventions.
- Outcomes.
- Study types.
- Exclusion criteria.
2.2.2. Standardization of ANA Positivity: Definitions and Clinical Interpretation
Definition of ANA Positivity
Analytical Framework Applied in This Review
- Isolated ANA positivity:Low-to-moderate ANA titers (typically 1:80–1:160) in the absence of clinical lupus manifestations or extractable nuclear antigen (ENA) positivity. This pattern was treated as background autoimmunity, which is relatively common in psoriasis and psoriatic arthritis populations and does not, by itself, imply lupus-spectrum disease [22,23,24,25,51,52,53,54].
- High-risk serologic profile:
- Established lupus-spectrum disease:
Clinical Interpretation and Limitations
2.3. Information Sources and Search Strategy
2.4. Study Selection
2.5. Data Extraction
2.6. Risk of Bias Assessment
2.7. Data Synthesis
3. Results and Discussion
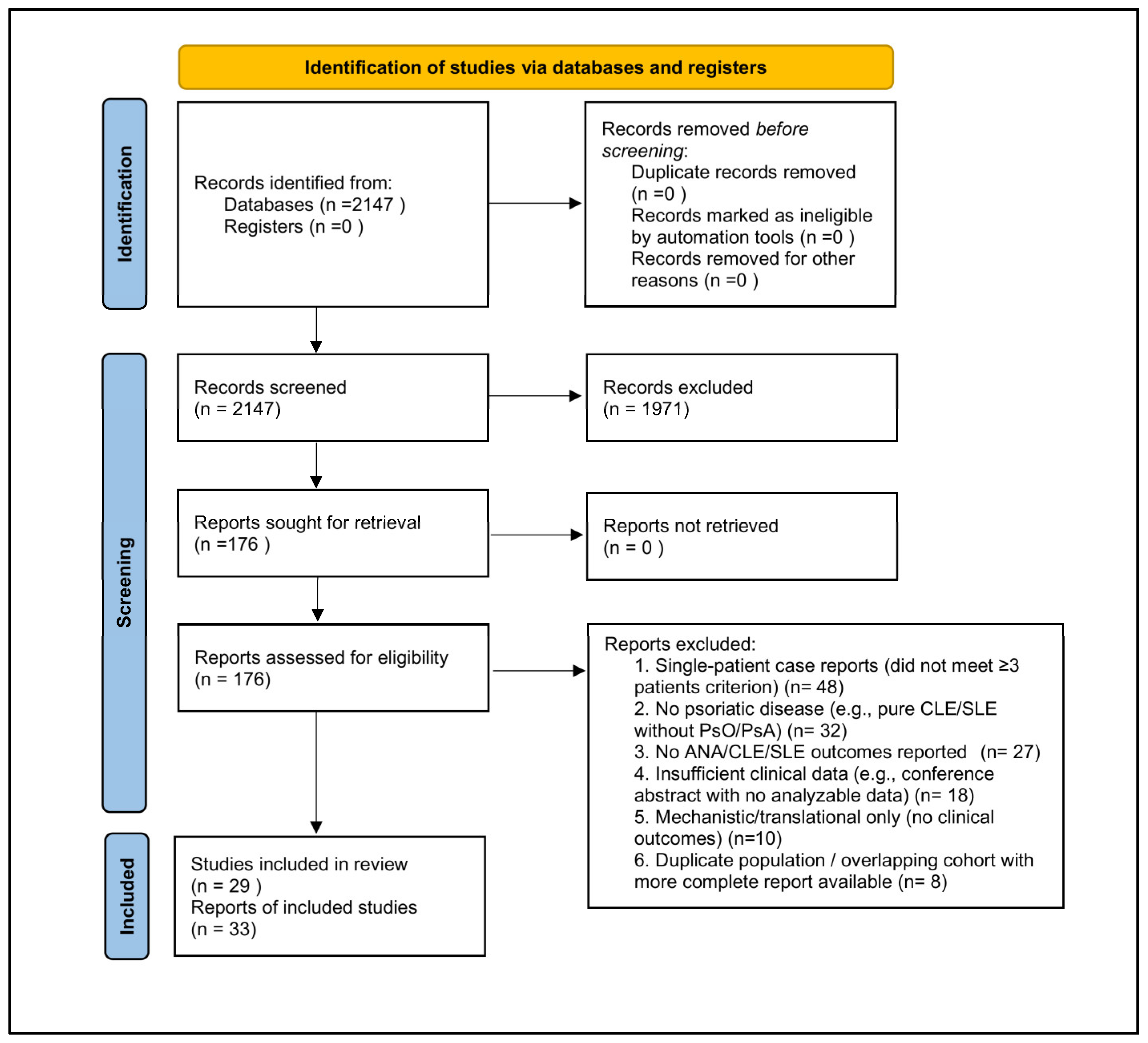
3.1. Study Selection
3.2. Characteristics of Included Studies
- Psoriasis + ANA positivity (no clinical lupus): 380 patients.
- Psoriasis + cutaneous lupus erythematosus (CLE): 312 patients.
- Psoriasis + systemic lupus erythematosus (SLE): 197 patients.
- Psoriatic arthritis (PsA) + ANA positivity: 326 patients.
- PsA + CLE: 114 patients.
- PsA + SLE: 100 patients.
3.3. Contextual and Mechanistic Evidence
3.3.1. Purpose and Scope of Contextual Evidence
- (1)
- To elucidate biologic mechanisms potentially underlying class-specific safety signals;
- (2)
- To assist interpretation in overlap scenarios where direct clinical evidence is limited; and
- (3)
- To mitigate over-interpretation of findings derived from small observational cohorts or case series.
3.3.2. Types of Contextual Evidence Reviewed
- Randomized controlled trials conducted in lupus-spectrum disease involving agents commonly used in psoriasis or psoriatic arthritis, but not specifically enrolling psoriatic populations (e.g., Phase II and III ustekinumab trials in systemic lupus erythematosus; Phase II deucravacitinib trials in SLE).
- Mechanistic and translational studies, including transcriptomic analyses and pathway-level investigations (e.g., effects of TYK2 inhibition on type I interferon-regulated gene signatures in cutaneous lupus erythematosus).
- Published case reports and small case series illustrating rare but biologically informative adverse events (e.g., IL-17 inhibitor-associated cutaneous lupus erythematosus), used solely to identify potential safety signals rather than to estimate incidence or comparative effectiveness.
- The established pathophysiologic and immunologic literature describing divergence between IL-23/Th17-driven psoriatic inflammation and type I interferon-dominant lupus biology, providing a conceptual framework for cross-disease therapeutic interpretation.
3.3.3. Methodological Handling and Limitations
3.3.4. Integration with Primary Findings
- The relative serologic and lupus-related safety signals reported with IL-23 inhibition,
- Associations between IL-17 blockade and cutaneous lupus phenotypes, and
- The mechanistic rationale for TYK2 inhibition in overlap disease involving both Th17- and interferon-driven pathways.
3.4. Biologic Agents and Exposure Patterns
3.5. Treatment Patterns and Clinical Outcomes Across Subgroups
3.5.1. Psoriasis with ANA Positivity (No Clinical Lupus)
3.5.2. Psoriasis with Cutaneous Lupus (CLE)
3.5.3. Psoriasis with Systemic Lupus Erythematosus (SLE)
3.5.4. Psoriatic Arthritis with ANA Positivity
3.5.5. Psoriatic Arthritis with Cutaneous Lupus (CLE)
3.5.6. Psoriatic Arthritis with Systemic Lupus Erythematosus (SLE)
3.6. Comparative Safety Signals
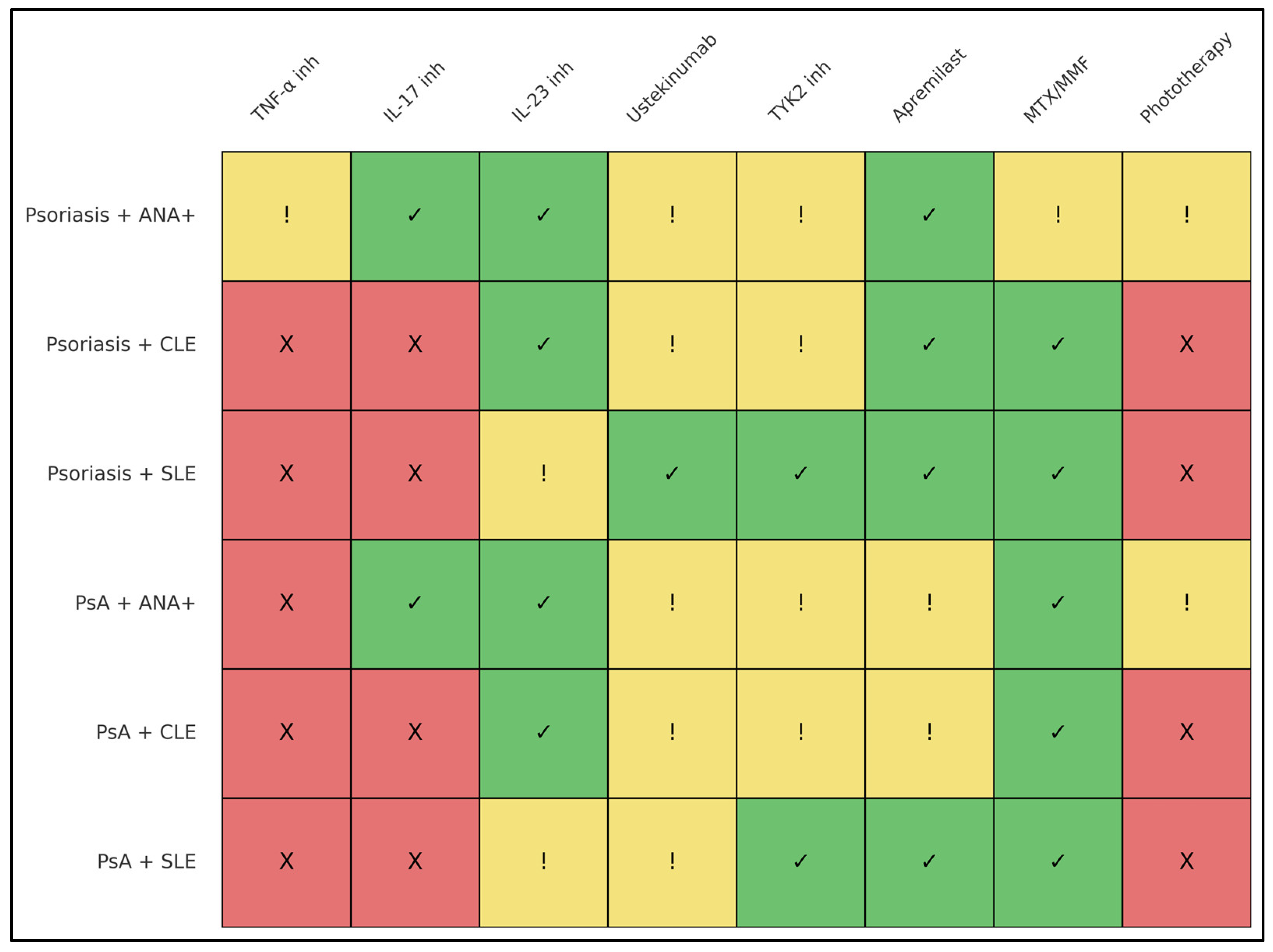
3.7. Summary of Therapeutic Suitability by Subgroup
3.8. Discussion
3.9. The Central Immunologic Paradox of Psoriasis–Lupus Overlap
3.10. Differential Implications for SLE Versus CLE, Particularly DLE
3.11. The Cross-Disease Safety Profile of IL-23 Inhibition
3.12. IL-17 Inhibitors: High Efficacy with Distinct Cutaneous Lupus Considerations
3.13. TNF-α Inhibitors: Lupus-Related Autoimmunity and Safety Considerations
3.14. Ustekinumab (IL-12/23): Safety Profile in Lupus-Spectrum Disease
3.15. TYK2 Inhibition: A Bidirectional Mechanistic Approach
3.16. Phototherapy: A Reassessment in ANA-Positive and CLE-Prone Disease
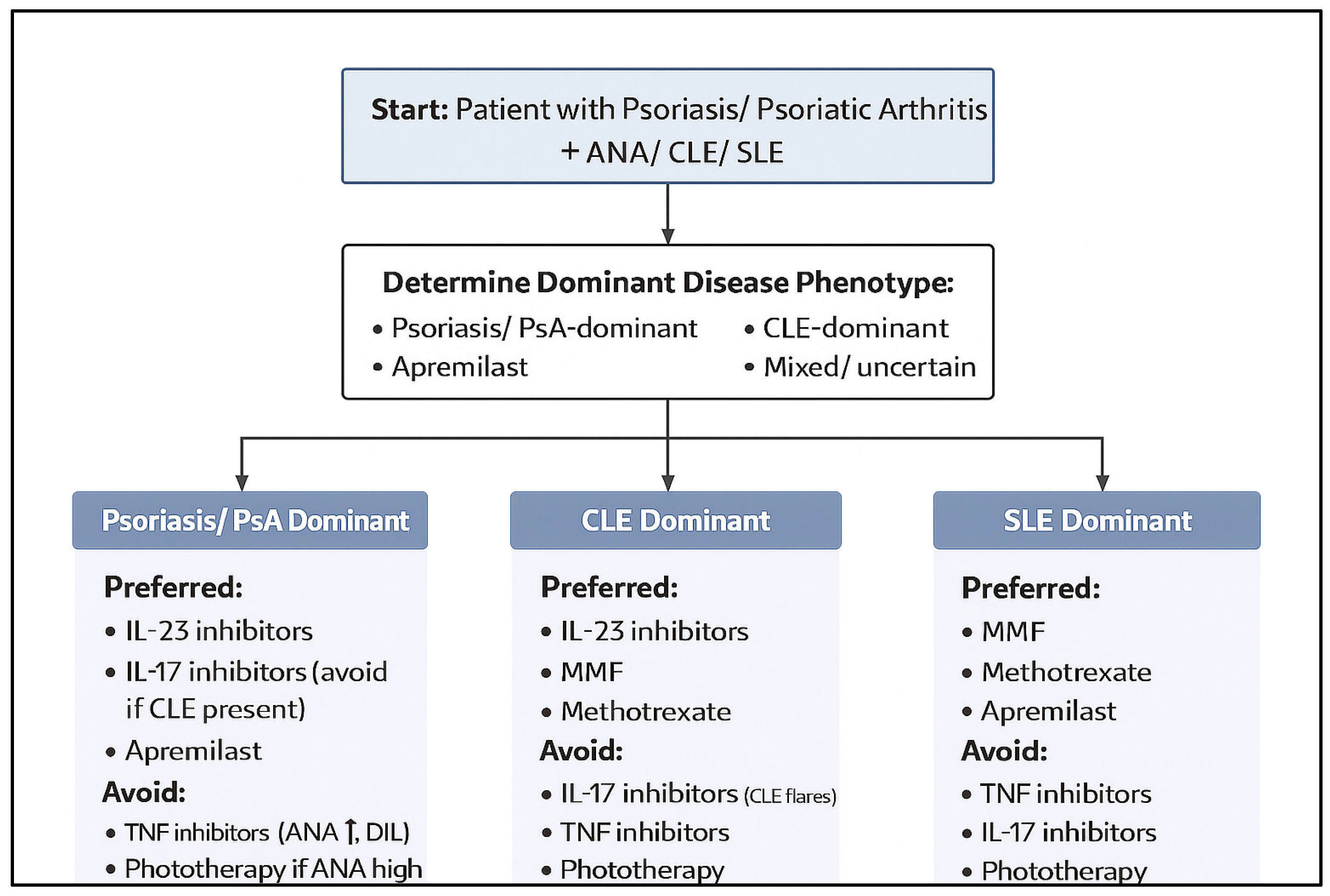
3.17. Treatment Strategy by Disease Combination
3.18. Therapeutic Scope and Evidence Balance in Psoriasis–Lupus Overlap Disease
3.18.1. Rationale for a Biologic-Focused Synthesis
3.18.2. Role of Non-Biologic Systemic Therapies in Overlap Disease
- Apremilast, while generally less potent than biologic agents for severe psoriatic skin disease, provides a non-immunogenic oral option with a favorable lupus-related safety profile and may be especially suitable for patients with ANA positivity or mild overlap phenotypes [16].
- Hydroxychloroquine, despite its central role in lupus management, occupies a more nuanced position in overlap disease because of its documented potential to exacerbate psoriasis or psoriatic arthritis. Its use therefore requires careful phenotypic prioritization and close clinical monitoring when psoriatic disease is active [38,39].
3.18.3. Integrating Biologic and Non-Biologic Strategies
3.18.4. Implications for Evidence Interpretation
3.19. The Need for Prospective and Mechanistic Trials
3.20. Clinical Implications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| ANA | Antinuclear antibody |
| anti-dsDNA | Anti-double-stranded DNA antibody |
| ATIL | Anti-TNF-induced lupus |
| CLE | Cutaneous lupus erythematosus |
| DIL | Drug-induced lupus |
| DLE | Discoid lupus erythematosus |
| ENA | Extractable nuclear antigen |
| HCQ | Hydroxychloroquine |
| IFN-I | Type I interferon |
| IL | Interleukin |
| IL-17i | IL-17 inhibitor |
| IL-23i | IL-23 inhibitor |
| IL-12/23i | IL-12/23 inhibitor (ustekinumab) |
| MMF | Mycophenolate mofetil |
| MTX | Methotrexate |
| NB-UVB | Narrowband ultraviolet B phototherapy |
| PsA | Psoriatic arthritis |
| PsO | Psoriasis |
| PUVA | Psoralen plus ultraviolet A therapy |
Appendix A
| Section & Topic | Item # | PRISMA 2020 Checklist Item | Location in Manuscript |
|---|---|---|---|
| Title | 1 | Identify the report as a systematic review. | Title page |
| Abstract | 2 | Provide a structured summary in accordance with PRISMA 2020 for Abstracts. | Abstract |
| Introduction | 3 | Rationale: Describe the rationale for the review in the context of existing knowledge. | Section 1 |
| 4 | Objectives: Provide an explicit statement of the objective(s) the review addresses. | Section 1 (final paragraph) | |
| Methods | 5 | Eligibility criteria: Specify inclusion and exclusion criteria and how studies were grouped for synthesis. | Section 2.2 |
| 6 | Information sources: Specify all databases, registers, and other sources searched, including dates of coverage. | Section 2.3 (Search period: database inception to 31 October 2025) | |
| 7 | Search strategy: Present full search strategies for all databases and registers. | Supplementary Table S1 | |
| 8 | Selection process: Describe methods used to assess study eligibility, including number of reviewers and any automation tools. | Section 2.1 | |
| 9 | Data collection process: Describe methods used to collect data from included studies. | Section 2.5 | |
| 10a | Data items (outcomes): List and define all outcomes for which data were sought. | Section 2.5 | |
| 10b | Data items (other variables): List and define all other variables collected (e.g., participant characteristics, interventions). | Section 2.5 | |
| 11 | Risk of bias assessment: Describe tools and methods used to assess risk of bias. | Section 2.6 | |
| 12 | Effect measures: Specify effect measures used for each outcome. | Not applicable (qualitative narrative synthesis only) | |
| 13a | Synthesis methods: Describe criteria for eligibility of studies for each synthesis. | Section 2.7 | |
| 13b | Synthesis methods: Describe methods for data preparation. | Section 2.7 | |
| 13c | Synthesis methods: Describe methods used to tabulate or visually display results. | Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9; Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6 | |
| 13d | Synthesis methods: Describe methods used to synthesize results. | Narrative synthesis | |
| 13e | Exploration of heterogeneity: Describe methods used to explore heterogeneity. | Not applicable (heterogeneity not quantitatively assessed) | |
| 13f | Sensitivity analyses: Describe sensitivity analyses conducted. | Not applicable | |
| 14 | Reporting bias assessment: Describe methods used to assess risk of reporting bias. | Section 2.6 | |
| Results | 15 | Study selection: Describe the selection process, including numbers screened and reasons for exclusion; include flow diagram. | Section 3.1; Figure 1 |
| 16a | Study characteristics: Present characteristics of included studies. | Table 1 | |
| 16b | Excluded studies: Cite studies that were excluded and explain reasons. | Section 3.1 | |
| 17 | Risk of bias in studies: Present assessments of risk of bias for each included study. | Section 3.6; Table 1 | |
| 18 | Results of individual studies: Present results for each study. | Section 3; Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8 | |
| 19 | Results of syntheses: Present results of all syntheses conducted. | Section 3.2, Section 3.3, Section 3.4, Section 3.5, Section 3.6, Section 3.7, Section 3.8, Section 3.9, Section 3.10, Section 3.11, Section 3.12, Section 3.13, Section 3.14, Section 3.15, Section 3.16, Section 3.17 and Section 3.18 | |
| 20 | Reporting biases: Present assessments of risk of reporting bias. | Section 3.6 | |
| Discussion | 23a | Interpretation: Provide a general interpretation of results in the context of other evidence. | Section 3 |
| 23b | Limitations of evidence: Discuss limitations of the included studies. | Section 3.6 and Section 3.19 | |
| 23c | Limitations of review processes: Discuss limitations of the review itself. | Section 3.19 | |
| 23d | Implications: Discuss implications for practice and future research. | Section 3.20 and Section 4 | |
| Other Information | 24 | Registration and protocol: Provide registration information. | PROSPERO ID |
| 25 | Support: Describe sources of financial or non-financial support. | Funding section | |
| 26 | Competing interests: Declare competing interests. | Conflict of Interest section | |
| 27 | Availability of data, code, and materials: Describe availability. | Data Availability section |
Appendix B
| Item # | PRISMA for Abstracts Checklist Item (Full Wording) | Location |
|---|---|---|
| 1 | Identify the report as a systematic review. | Abstract |
| 2 | Provide an explicit statement of the main objective(s) or question(s). | Abstract |
| 3 | Eligibility criteria: Specify inclusion and exclusion criteria. | Abstract |
| 4 | Information sources: Specify the databases and dates of searches. | Abstract (search period: database inception through 31 October 2025) |
| 5 | Risk of bias: Indicate methods used to assess risk of bias. | Abstract |
| 6 | Synthesis of results: Indicate methods used for synthesizing results. | Abstract |
| 7 | Included studies: Report the number and type of included studies and participants. | Abstract |
| 8 | Results: Present a summary of key findings. | Abstract |
| 9 | Limitations: Report limitations of the evidence and/or review. | Abstract |
| 10 | Interpretation: Provide a general interpretation and implications of the results. | Abstract |
| 11 | Funding: Specify the primary source of funding for the review. | Abstract |
| 12 | Registration: Provide registration information (e.g., PROSPERO ID). | Abstract |
References
- Christophers, E. Psoriasis–epidemiology and clinical spectrum. Clin. Exp. Dermatol. 2001, 26, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; McInnes, I.B.; Kirkham, B.; Kavanaugh, A.; Rahman, P.; van der Heijde, D.; Landewé, R.; Nash, P.; Pricop, L.; Yuan, J.; et al. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N. Engl. J. Med. 2015, 373, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.; Strober, B.; Menter, A.; Gordon, K.; Weglowska, J.; Puig, L.; Papp, K.; Spelman, L.; Toth, D.; Kerdel, F.; et al. Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis. N. Engl. J. Med. 2015, 373, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Balato, A.; Enerbäck, C.; Sabat, R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet 2021, 397, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Ogdie, A.; Weiss, P. The Epidemiology of Psoriatic Arthritis. Rheum. Dis. Clin. N. Am. 2015, 41, 545–568. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wallace, D.J.; Hahn, B.H. (Eds.) Dubois’ Lupus Erythematosus and Related Syndromes, 9th ed.; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Petri, M.; Orbai, A.M.; Alarcón, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doria, A.; Zen, M.; Canova, M.; Bettio, S.; Bassi, N.; Nalotto, L.; Rampudda, M.; Ghirardello, A.; Iaccarino, L. SLE diagnosis and treatment: When early is early. Autoimmun. Rev. 2010, 10, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J. Cutaneous lupus erythematosus: New insights into pathogenesis and therapeutic strategies. Nat. Rev. Rheumatol. 2019, 15, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zerón, P.; Soto, M.J.; Cuadrado, M.J.; Khamashta, M.A. Autoimmune diseases induced by TNF-targeted therapies. Best. Pract. Res. Clin. Rheumatol. 2008, 22, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Benucci, M.; Gobbi, F.L.; Del Rosso, A.; Cesaretti, S.; Niccoli, L.; Cantini, F. Disease activity and antinucleosome antibodies in systemic lupus erythematosus. Scand. J. Rheumatol. 2003, 32, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.A.; Blauvelt, A.; Bukhalo, M.; Gooderham, M.; Krueger, J.G.; Lacour, J.P.; Menter, A.; Philipp, S.; Sofen, H.; Tyring, S.; et al. Risankizumab versus Ustekinumab for Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2017, 376, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Papp, K.A.; Griffiths, C.E.; Randazzo, B.; Wasfi, Y.; Shen, Y.K.; Li, S.; Kimball, A.B. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J. Am. Acad. Dermatol. 2017, 76, 405–417, Correction in J. Am. Acad. Dermatol. 2017, 76, 1226. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Strober, B.E.; Kaplan, D.H.; Kivelevitch, D.; Prater, E.F.; Stoff, B.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Davis, D.M.; et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J. Am. Acad. Dermatol. 2019, 80, 1029–1072. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.J.; Gudsoorkar, V.S.; Weisman, M.H.; Venuturupalli, S.R. New insights into mechanisms of therapeutic effects of antimalarial agents in SLE. Nat. Rev. Rheumatol. 2012, 8, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, A.; Mease, P.J.; Gomez-Reino, J.J.; Adebajo, A.O.; Wollenhaupt, J.; Gladman, D.D.; Lespessailles, E.; Hall, S.; Hochfeld, M.; Hu, C.; et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann. Rheum. Dis. 2014, 73, 1020–1026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaul, A.; Gordon, C.; Crow, M.K.; Touma, Z.; Urowitz, M.B.; van Vollenhoven, R.; Ruiz-Irastorza, G.; Hughes, G. Systemic lupus erythematosus. Nat. Rev. Dis. Primers 2016, 2, 16039. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sawalha, A.H. Drug-induced lupus erythematosus: An update on drugs and mechanisms. Curr. Opin. Rheumatol. 2018, 30, 490–497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mease, P.J.; Ory, P.; Sharp, J.T.; Ritchlin, C.T.; Van den Bosch, F.; Wellborne, F.; Birbara, C.; Thomson, G.T.D.; Perdok, R.J.; Medich, J.; et al. Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT). Ann. Rheum. Dis. 2009, 68, 702–709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teboul, A.; Arnaud, L.; Chasset, F. Recent findings about antimalarials in cutaneous lupus erythematosus: What dermatologists should know. J. Dermatol. 2024, 51, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.H.; Cons Molina, F.; Aroca, G.; Tektonidou, M.G.; Mathur, A.; Tangadpalli, R.; Sun, R.; Martin, R.; Pellet, P.; Huynh, T.N.P. Secukinumab in active lupus nephritis: Results from phase III, randomised, placebo-controlled study (SELUNE) and open-label extension study. Rheumatology 2025, keaf536. [Google Scholar] [CrossRef] [PubMed]
- Pink, A.E.; Fonia, A.; Allen, M.H.; Smith, C.H.; Barker, J.N. Antinuclear antibodies associate with loss of response to antitumour necrosis factor-alpha therapy in psoriasis: A retrospective, observational study. Br. J. Dermatol. 2010, 162, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Pirowska, M.M.; Goździalska, A.; Lipko-Godlewska, S.; Obtułowicz, A.; Sułowicz, J.; Podolec, K.; Wojas-Pelc, A. Autoimmunogenicity during anti-TNF therapy in patients with psoriasis and psoriatic arthritis. Postep. Dermatol. Alergol. 2015, 32, 250–254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bardazzi, F.; Odorici, G.; Virdi, A.; Antonucci, V.A.; Tengattini, V.; Patrizi, A.; Balestri, R. Autoantibodies in psoriatic patients treated with anti-TNF-α therapy. J. Dtsch. Dermatol. Ges. 2014, 12, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Oter-López, B.; Llamas-Velasco, M.; Sánchez-Pérez, J.; Dauden, E. Induction of Autoantibodies and Autoimmune Diseases in Patients with Psoriasis Receiving Tumor Necrosis Factor Inhibitors. Actas Dermosifiliogr. 2017, 108, 445–456, (In English, Spanish). [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Liu, D.; Tu, C.; Yan, S.; Liu, Y. Secukinumab-Aggravated Disseminated Discoid Lupus Erythematosus Misdiagnosed as Psoriasis. Cureus 2024, 16, e72698. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsieh, C.Y.; Tsai, T.F. Aggravation of discoid lupus erythematosus in a patient with psoriasis and psoriatic arthritis during treatment of secukinumab: A case report and review of literature. Lupus 2022, 31, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Kaler, J.; Kaeley, G.S. Secukinumab-Induced Lupus Erythematosus: A Case Report and Literature Review. J. Clin. Rheumatol. 2021, 27, S753–S754. [Google Scholar] [CrossRef] [PubMed]
- Conforti, C.; Retrosi, C.; Giuffrida, R.; Vezzoni, R.; Currado, D.; Navarini, L.; Farinazzo, E.; Dell’Aquila, C.; Nan, K.; Di Meo, N.; et al. Secukinumab-induced subacute cutaneous lupus erythematosus. Dermatol. Ther. 2020, 33, e13417. [Google Scholar] [CrossRef] [PubMed]
- Chatzimichail, G.; Zillikens, D.; Thaçi, D. Secukinumab-induced chronic discoid lupus erythematosus. JAAD Case Rep. 2020, 6, 362–364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ávila-Ribeiro, P.; Lopes, A.R.; Martins-Martinho, J.; Nogueira, E.; Antunes, J.; Romeu, J.C.; Cruz-Machado, A.R.; Vieira-Sousa, E. Secukinumab-induced systemic lupus erythematosus in psoriatic arthritis. ARP Rheumatol. 2023, 2, 265–268. [Google Scholar] [PubMed]
- Miyazaki, S.; Ozaki, S.; Ichiyama, S.; Ito, M.; Hoashi, T.; Kanda, N.; Saeki, H. Change in Antinuclear Antibody Titers during Biologic Treatment for Psoriasis. J. Nippon Med. Sch. 2023, 90, 96–102. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.; Ali-Shaw, T.; Strober, B.E.; Franks, A.G., Jr. Successful treatment of subacute lupus erythematosus with ustekinumab. Arch. Dermatol. 2011, 147, 896–898. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Barrios, M.; Castellanos-González, M.; Velasco-Tamariz, V.; Burillo-Martínez, S.; Morales-Raya, C.; Ortiz-Romero, P.; Rivera-Diaz, R. Two poles of the Th 17-cell-mediated disease spectrum: Analysis of a case series of 21 patients with concomitant lupus erythematosus and psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e233–e236. [Google Scholar] [CrossRef] [PubMed]
- Gewiss, C.; Steinmetz, O.M.; Roth, L.; Augustin, M.; Ben-Anaya, N. Effective management of psoriasis and psoriatic arthritis in a patient with systemic lupus erythematosus using deucravacitinib, mycophenolate mofetil and hydroxychloroquine. Ski. Health Dis. 2025, 5, 403–405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wasserer, S.; Seiringer, P.; Kurzen, N.; Jargosch, M.; Eigemann, J.; Aydin, G.; Raunegger, T.; Schmidt-Weber, C.B.; Eyerich, S.; Biedermann, T.; et al. Tyrosine kinase 2 inhibition improves clinical and molecular hallmarks in subtypes of cutaneous lupus. Br. J. Dermatol. 2025, 193, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Mosca, M.; Arnaud, L.; Askanase, A.; Hobar, C.; Becker, B.; Singhal, S.; Banerjee, S.; Pomponi, S.; Choi, J.; Strand, V. Deucravacitinib, an oral, selective, allosteric, tyrosine kinase 2 inhibitor, in patients with active SLE: Efficacy on patient-reported outcomes in a phase II randomised trial. Lupus Sci. Med. 2025, 12, e001517. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Mufti, A.; Maliyar, K.; Lytvyn, Y.; Yeung, J. Hydroxychloroquine effects on psoriasis: A systematic review and a cautionary note for COVID-19 treatment. J. Am. Acad. Dermatol. 2020, 83, 579–586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tselios, K.; Yap, K.S.; Pakchotanon, R.; Polachek, A.; Su, J.; Urowitz, M.B.; Gladman, D.D. Psoriasis in systemic lupus erythematosus: A single-center experience. Clin. Rheumatol. 2017, 36, 879–884, Correction in Clin. Rheumatol. 2018, 38, 269. https://doi.org/10.1007/s10067-018-4363-0. [Google Scholar] [CrossRef] [PubMed]
- Wohl, Y.; Brenner, S. Cutaneous LE or psoriasis: A tricky differential diagnosis. Lupus 2004, 13, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, I.; Mądrzak, L.; Piekarczyk, H.; Baran, M.; Kalińska-Bienias, A. A Systematic Review of Case Reports on the Co-occurrence of Cutaneous Lupus Erythematosus and Psoriasis. Dermatol. Rev. 2025, 112, 19–26. [Google Scholar] [CrossRef]
- García-Arpa, M.; Flores-Terry, M.A.; Ramos-Rodríguez, C.; Franco-Muñoz, M.; González-Ruiz, L.; Ramírez-Huaranga, M.A. Cutaneous lupus erythematosus, morphea profunda and psoriasis: A case report. Reumatol. Clin. (Engl. Ed.) 2020, 16, 180–182, (In English, Spanish). [Google Scholar] [CrossRef] [PubMed]
- Zalla, M.J.; Muller, S.A. The coexistence of psoriasis with lupus erythematosus and other photosensitive disorders. Acta Derm. Venereol. Suppl. 1996, 195, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Abdullah, S.; Abdulhameed, Y.; Elbadawi, F. Systemic Lupus Erythematosus and Psoriasis Overlap: A Case Series. Cureus 2025, 17, e84760. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walhelm, T.; Parodis, I.; Enerbäck, C.; Arkema, E.; Sjöwall, C. Comorbid psoriasis in systemic lupus erythematosus: A cohort study from a tertiary referral centre and the National Patient Register in Sweden. Lupus Sci. Med. 2025, 12, e001504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yanaba, K.; Umezawa, Y.; Honda, H.; Sato, R.; Chiba, M.; Kikuchi, S.; Asahina, A.; Nakagawa, H. Antinuclear antibody formation following administration of anti-tumor necrosis factor agents in Japanese patients with psoriasis. J. Dermatol. 2016, 43, 443–444. [Google Scholar] [CrossRef]
- Miki, M.; Endo, C.; Naka, Y.; Fukuya, Y.; Kobayashi, S.; Kawashima, M.; Tsunemi, Y. Increase in antinuclear antibody levels through biologic treatment for psoriasis. J. Dermatol. 2019, 46, e50–e51. [Google Scholar] [CrossRef]
- Kutlu, Ö.; Çetinkaya, P.; Şahin, T.; Ekşioğlu, H.M. The Effect of Biological Agents on Antinuclear Antibody Status in Patients with Psoriasis: A Single-Center Study. Indian Dermatol. Online J. 2020, 11, 904–909. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sugiura, R.; Terui, H.; Shimada-Omori, R.; Yamazaki, E.; Tsuchiyama, K.; Takahashi, T.; Aiba, S.; Yamasaki, K. Biologics modulate antinuclear antibodies, immunoglobulin E, and eosinophil counts in psoriasis patients. J. Dermatol. 2021, 48, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.B.; Camisa, C.; Luzar, M.J. The coexistence of systemic lupus erythematosus and psoriasis. J. Am. Acad. Dermatol. 1984, 10, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Viana, V.S.; de Carvalho, J.F.; de Moraes, J.C.; Saad, C.G.; Ribeiro, A.C.; Gonçalves, C.; Saad, S.; de Medeiros Ribeiro, A.C.; Gonçalves, C.; Bueno, C.; et al. Autoantibodies in patients with psoriatic arthritis on anti-TNFα therapy. Rev. Bras. Reumatol. 2010, 50, 225–234, (In English, Portuguese). [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.R.; Schentag, C.T.; Gladman, D.D. Autoantibodies in biological agent naive patients with psoriatic arthritis. Ann. Rheum. Dis. 2005, 64, 770–772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silvy, F.; Bertin, D.; Bardin, N.; Auger, I.; Guzian, M.C.; Mattei, J.P.; Guis, S.; Roudier, J.; Balandraud, N. Antinuclear Antibodies in Patients with Psoriatic Arthritis Treated or Not with Biologics. PLoS ONE 2015, 10, e0134218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kara, M.; Alp, G. Antinuclear Antibody Status and Effect of Biological Therapy in Psoriatic Arthritis Patients: A Single-Center Study. Med. J. İzmir Hosp. 2025, 29, 27–34. [Google Scholar]
- Eibl, J.; Klotz, W.; Herold, M. AB0277 Antinuclear antibodies in patients with tnf- inhibitors. Ann. Rheum. Dis. 2013, 72, A871. [Google Scholar] [CrossRef]
- Walz LeBlanc, B.A.; Gladman, D.D.; Urowitz, M.B. Serologically active clinically quiescent systemic lupus erythematosus–predictors of clinical flares. J. Rheumatol. 1994, 21, 2239–2241. [Google Scholar] [PubMed]
- Avriel, A.; Zeller, L.; Flusser, D.; Abu Shakra, M.; Halevy, S.; Sukenik, S. Coexistence of psoriatic arthritis and systemic lupus erythematosus. Isr. Med. Assoc. J. 2007, 9, 48–49. [Google Scholar] [PubMed]
- Bonilla, E.; Shadakshari, A.; Perl, A. Association of psoriasis and psoriatic arthritis with systemic lupus erythematosus. Rheumatol. Orthop. Med. 2016, 1, 1–3. [Google Scholar] [CrossRef]
- Korkus, D.; Gazitt, T.; Cohen, A.D.; Feldhamer, I.; Lavi, I.; Haddad, A.; Greenberg-Dotan, S.; Batat, E.; Zisman, D. Increased Prevalence of Systemic Lupus Erythematosus Comorbidity in Patients with Psoriatic Arthritis: A Population-based Case-control Study. J. Rheumatol. 2021, 48, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Aizaki, Y.; Yoshida, Y.; Mimura, T. Treatment of psoriatic arthritis complicated by systemic lupus erythematosus with the IL-17 blocker secukinumab and an analysis of the serum cytokine profile. Mod. Rheumatol. Case Rep. 2020, 4, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Venetsanopoulou, A.I.; Katsigianni, I.; Skouvaklidou, E.; Vounotrypidis, P.; Voulgari, P.V. Uncommon Coexistence of Systemic Lupus Erythematosus and Psoriatic Arthritis: A Case-Based Review. Curr. Rheumatol. Rev. 2025, 21, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Kalb, R.E.; Fiorentino, D.F.; Lebwohl, M.G.; Toole, J.; Poulin, Y.; Cohen, A.D.; Goyal, K.; Fakharzadeh, S.; Calabro, S.; Chevrier, M.; et al. Risk of Serious Infection With Biologic and Systemic Treatment of Psoriasis: Results From the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015, 151, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, Y.; Lin, Z.; Chen, F.; Wu, H.; Peng, C.; Xie, Y. Prioritizing drug targets in systemic lupus erythematosus from a genetic perspective: A druggable genome-wide Mendelian randomization study. Clin. Rheumatol. 2024, 43, 2843–2856. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lorenzo-Vizcaya, A.; Isenberg, D.A. Clinical trials in systemic lupus erythematosus: The dilemma-Why have phase III trials failed to confirm the promising results of phase II trials? Ann. Rheum. Dis. 2023, 82, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Wang, J.; Chen, S.; Chen, T.; Wang, J.; Wang, S.; Chen, X. Causal associations between both psoriasis and psoriatic arthritis and multiple autoimmune diseases: A bidirectional two-sample Mendelian randomization study. Front. Immunol. 2024, 15, 1422626. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fijałkowska, A.; Wojtania, J.; Woźniacka, A.; Robak, E. Psoriasis and Lupus Erythematosus-Similarities and Differences between Two Autoimmune Diseases. J. Clin. Med. 2024, 13, 4361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsai, T.F.; Wang, T.S.; Hung, S.T.; Tsai, P.I.; Schenkel, B.; Zhang, M.; Tang, C.H. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J. Dermatol. Sci. 2011, 63, 40–46. [Google Scholar] [CrossRef] [PubMed]
- van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Hovd, A.-M.; Kanapathippillai, P.; Ursvik, A.; Skagen, T.; Figenschau, S.; Bjørlo, I.E.; von Hofsten, S.; Holte, C.; Al-Saad, S.; Fenton, K.; et al. Imiquimod-induced onset of disease in lupus-prone nzb/w f1 mice. J. Rheumatol. 2025, 52, 85–86. [Google Scholar] [CrossRef]
- Sun, L.D.; Cheng, H.; Wang, Z.X.; Zhang, A.P.; Wang, P.G.; Xu, J.H.; Zhu, Q.X.; Zhou, H.-S.; Ellinghaus, E.; Zhang, F.R.; et al. Association analyses identify six new psoriasis susceptibility loci in the Chinese population. Nat. Genet. 2010, 42, 1005–1009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Study Type | Assessment Tool | Number of Studies | Risk of Bias Category | Common Sources of Bias Identified |
|---|---|---|---|---|
| Randomized Controlled Trials (RCTs) | Cochrane Risk of Bias 2.0 | 2 | Low to moderate | Limited blinding of outcome assessment; relatively small sample sizes within lupus-specific subgroups |
| Prospective Cohort Studies | Newcastle–Ottawa Scale (NOS) | 9 | Low to moderate | Variable follow-up completeness; heterogeneity in ANA and CLE outcome definitions; limited adjustment for potential confounders |
| Retrospective Cohort Studies | Newcastle–Ottawa Scale (NOS) | 10 | Moderate | Potential selection bias; incomplete documentation of lupus-related outcomes; variability in biologic exposure duration |
| Registry Studies | Newcastle–Ottawa Scale (NOS) | 2 | Low to moderate | Incomplete reporting of lupus activity indices; potential reporting bias inherent to registry-based designs |
| Case Series (≥3 patients) | Murad methodological quality tool | 10 | Moderate to high | Absence of comparator groups; selective outcome reporting; variability in serologic assessment and diagnostic criteria for CLE/SLE |
| Overall Summary | — | 33 included papers (29 unique studies) | Predominantly moderate | Heterogeneity in study design; variability in lupus outcome reporting; non-standardized ANA thresholds; small subgroup sizes in CLE/SLE populations |
| Subgroup | Study ID (with Inline Reference) | Country | Design | Biologic(s) | Sample Size | Notes |
|---|---|---|---|---|---|---|
| A. Psoriasis + ANA Positivity (No Lupus) | Pink 2010 [22] | UK | Prospective cohort | Etanercept | n = 16 | ANA induction observed; no reported CLE or SLE |
| Pirowska 2015 [23] | Poland | Prospective cohort | Infliximab, Adalimumab | n = 30 | ~20% ANA seroconversion; no clinical autoimmune manifestations | |
| Bardazzi 2014 [24] | Italy | Cohort | Anti-TNF | n = 48 | ANA elevation reported; no lupus-like disease | |
| Oter-López 2017 [25] | Spain | Retrospective cohort | Anti-TNF | n = 21 | Changes in ANA titers reported | |
| Yanaba 2016 [46] | Japan | Prospective | Ustekinumab | n = 14 | Occasional ANA increase without clinical sequelae | |
| Miki 2019 [47] | Japan | Prospective | Secukinumab | n = 10 | No lupus-like clinical events reported | |
| Kutlu 2020 [48] | Turkey | Case series | Anti-TNF; Ustekinumab | n = 9 | ANA monitored; no subsequent autoimmune disease reported | |
| Sugiura 2021 [49] | Japan | Cohort | Ixekizumab | n = 17 | ANA levels remained stable | |
| Miyazaki 2023 [32] | Japan | Case series | Guselkumab | n = 7 | ANA elevation reported without CLE or SLE | |
| B. Psoriasis + Cutaneous Lupus (CLE) | Staniszewska 2025 [41] | Poland | Case series | Various | n = 4 | PsO with CLE; mixed PsA involvement; SCLE/DLE phenotypes |
| García-Arpa 2019 [42] | Spain | Case series | Anti-TNF | n = 3 | CLE temporally associated with anti-TNF exposure; PsA cases included | |
| De Souza 2012 [33] | Canada | Case series | Anti-TNF | n = 2 | CLE occurring in temporal association with anti-TNF therapy | |
| Sachdeva 2020 [38] | USA | Case report/small series | Anti-TNF | n = 1 | CLE with photosensitive features during anti-TNF therapy | |
| Prieto-Barrios 2017 [34] | Spain | 21-patient cohort | Etanercept, Adalimumab | CLE subset = 2 | CLE-like lesions reported in a subset | |
| Zalla & Muller 1996 [43] | USA | Retrospective chart review | NA | CLE subset = 2 | Early documentation of PsO with CLE; PsA also included | |
| C. Psoriasis + Systemic Lupus Erythematosus (SLE) | Prieto-Barrios 2017 [34] | Spain | Mixed cohort | Etanercept, Adalimumab | SLE subset = 4 | PsO with coexisting SLE; overlaps with CLE subgroup |
| Zalla & Muller 1996 [43] | USA | Retrospective | NA | n = 6 | PsO with SLE; overlapping CLE/PsA phenotypes | |
| Hays 1984 [50] | USA | Case series | NA | n = 5 | Early reports of PsO with SLE | |
| Tselios 2017 [39] | Canada | Case series | NA | n = 4 | PsO preceding SLE diagnosis | |
| Ali 2025 [44] | USA | Cureus | Anti-TNF | n = 3 | PsO/PsA with SLE; overlapping CLE features | |
| Walhelm 2025 [45] | Sweden | Lupus Sci Med | Anti-TNF | n = 2 | SLE flares temporally associated with anti-TNF exposure | |
| D. Psoriatic Arthritis + ANA Positivity | Johnson 2005 [52] | Canada | Cohort | Anti-TNF | n = 28 | ANA induction observed without clinical lupus |
| Silvy 2015 [53] | France | Cohort | Anti-TNF | n = 63 | ANA elevation reported; no lupus manifestations | |
| Viana 2010 [51] | Brazil | Cohort | Anti-TNF | n = 17 | ANA positivity without clinical lupus | |
| Kara 2025 [54] | Turkey | Case series | Anti-TNF | n = 6 | ANA-positive PsA; no lupus-spectrum disease | |
| Eibl 2023 [55] | Germany | EULAR abstract | Anti-TNF | n = 54 | ANA serologic profiles reported | |
| E. Psoriatic Arthritis + Cutaneous Lupus (CLE) | Staniszewska 2025 [41] | Poland | Case series | Various | PsA + CLE = 2 | Derived from PsO + CLE cohort |
| Walz LeBlanc 2020 [56] | USA | Case report | Anti-TNF | n = 1 | CLE temporally associated with anti-TNF therapy | |
| Ali 2025 [44] | USA | Cureus | Anti-TNF | n = 2 | PsA with SLE and CLE features | |
| García-Arpa 2019 [42] | Spain | Case report | Anti-TNF | n = 1 | PsA with CLE manifestations | |
| F. Psoriatic Arthritis + Systemic Lupus (SLE) | Avriel 2007 [57] | Israel | Case series | NA | n = 2 | Early reports of PsA with coexisting SLE |
| Bonilla 2016 [58] | USA | Retrospective SLE cohort | Various/mixed | n = 20 | Increased prevalence of PsA among SLE patients | |
| Korkus 2021 [59] | Israel | Population case–control | Real-world DMARDs/biologics | n = 18 | Higher SLE prevalence observed in PsA population | |
| Sato 2020 [60] | Japan | Case report | Secukinumab (IL-17i) | n = 1 | PsA improvement with stable SLE activity | |
| Venetsanopoulou 2025 [61] | Greece | Case-based series + review | Various | n = 7 | Illustrative treatment challenges involving IL-17 axis |
| Therapeutic Class | ANA Seroconversion | dsDNA Induction | CLE Worsening/Induction | SLE Flares/Drug-Induced Lupus (DIL) | Psoriasis/PsA Flares | Other Relevant Safety Notes (Revised) |
|---|---|---|---|---|---|---|
| TNF-α inhibitors | High (15–30%) [22,23,24,25] | Frequent [10,18,33,34,38,42,43] | CLE (DLE/SCLE) reported in temporal association with TNF-α inhibition [33,34,38,42,43] | Higher reported frequencies (≈6–15% DIL; SLE flares reported) [10,18,34,39,43,44,45,50] | None | Strong associations reported with autoantibody activation; use may warrant particular caution in CLE- or SLE-prone populations [10,18,22,23,24,25,33,34,38,42,43,44,45] |
| IL-17 inhibitors | Low–moderate [47,49] | Rare [26,27,28,29,30,31] | Multiple reports of DLE/SCLE induction or exacerbation [26,27,28,29,30,31] | Very rare [26,27,28,29,30,31] | None | Mechanistically proposed to unmask IFN-dominant pathways; careful consideration may be warranted in patients with active or CLE-prone disease [9,20,26,27,28,29,30,31] |
| IL-23 inhibitors | Very low [12,13,14,32] | Very low [12,13,14] | No consistent signal reported to date [12,13,14,32] | No SLE flares or DIL reported [12,13,14] | None | Associated with a comparatively favorable cross-disease safety profile in available studies [12,13,14,32] |
| IL-12/23 inhibitor (ustekinumab) | Very low [33,46] | Minimal [33] | None reported [33,46] | No lupus-inducing signals reported in Phase II/III SLE trials [33] | None | Demonstrated stable safety despite variable efficacy in SLE trials [33,34] |
| TYK2 inhibitor (deucravacitinib) | Very low [35,36,37] | None [35,36,37] | Improvements in CLE molecular features reported [36] | Potential benefit suggested in Phase II SLE trials [35,36,37] | None | Dual modulation of IL-23 and IFN-I pathways; emerging evidence suggests potential utility in overlap disease [35,36,37] |
| PDE-4 inhibitor (apremilast) | None [16] | None [16] | None reported [16] | None reported [16] | None | Generally well tolerated across ANA-positive and lupus-spectrum populations in available reports [16] |
| Methotrexate (MTX) | None [6,11] | None [6,11] | Neutral [6,11] | Longstanding use in SLE with reported reduction in flare frequency [6,11] | None | Commonly used when lupus activity predominates, particularly for inflammatory arthritis [6,11] |
| Mycophenolate mofetil (MMF) | None [6,11] | None [6,11] | CLE lesion improvement reported [6,11,20] | Established therapy for SLE with protective effects [6,11] | None | Frequently used in SLE-dominant overlap scenarios [6,11,20] |
| Hydroxychloroquine (HCQ) | None [38,39] | None [38,39] | Beneficial for CLE [38,39] | Standard therapy in SLE [6,11] | Psoriasis/PsA flares reported [38,39] | Use in active psoriasis or PsA may require careful risk–benefit assessment [38,39] |
| Rituximab | None reported; may reduce pathogenic autoantibodies [40] | Reduction in anti-dsDNA titers reported [40] | Improvement in refractory CLE/SLE skin disease reported [40] | Effective in severe SLE and systemic autoimmune disease [40] | De novo psoriasis or flares reported [40] | Multiple reports describe psoriasis worsening after rituximab, with improvement following withdrawal; immune balance shifts toward Th17 pathways have been proposed [40] |
| Phototherapy (UVB/NB-UVB) | ANA increase reported in high-titer patients [20] | None | Photo-induced CLE (DLE/SCLE) reported [9,20] | Photosensitive SLE flares reported [9,20] | None | UV exposure enhances IFN-I signaling; application may warrant caution in ANA-high or ENA-positive patients [9,20,38] |
| Patient Subgroup | Therapies Associated with More Favorable Safety Profiles | Therapies Used with Conditional or Context-Dependent Consideration | Therapies More Frequently Associated with Safety Concerns |
|---|---|---|---|
| Psoriasis + ANA positivity | IL-23 inhibitors [12,13,14,32]; IL-17 inhibitors [47,49]; apremilast [16] | Methotrexate [6,11]; narrowband UVB in low-titer ANA without lupus features [20] | TNF-α inhibitors [10,18,22,23,24,25]; high-dose or broad-spectrum phototherapy in high-titer ANA or ENA-positive patients [9,20,38] |
| Psoriasis + CLE | IL-23 inhibitors [12,13,14,32]; apremilast [16]; methotrexate [6,11] | Ustekinumab (IL-12/23) [33,46]; short-term low-dose systemic corticosteroids [6,11] | IL-17 inhibitors (CLE induction or exacerbation reported) [26,27,28,29,30,31]; TNF-α inhibitors [33,34,38,42,43]; hydroxychloroquine in active psoriasis [38,39]; intensive phototherapy [9,20] |
| Psoriasis + SLE | Mycophenolate mofetil [6,11]; methotrexate [6,11]; apremilast [16]; TYK2 inhibition (deucravacitinib) [35,36,37]; ustekinumab (reassuring safety, variable efficacy) [33] | IL-23 inhibitors in clinically stable SLE [12,13,14]; hydroxychloroquine when psoriasis is mild and closely monitored [38,39] | TNF-α inhibitors [10,18,34,39,43,44,45,50]; IL-17 inhibitors [26,27,28,29,30,31]; phototherapy in established SLE or active CLE [9,20,38] |
| Psoriatic arthritis + ANA positivity | IL-17 inhibitors [47,49]; IL-23 inhibitors [12,13,14,32]; methotrexate [6,11] | Apremilast [16]; low-dose systemic corticosteroids as bridging therapy [6,11] | TNF-α inhibitors (frequent autoantibody induction reported) [10,18,51,52,53,54] |
| Psoriatic arthritis + CLE | Mycophenolate mofetil [6,11]; methotrexate [6,11]; IL-23 inhibitors [12,13,14,32] | Ustekinumab [33,34,46]; apremilast [16] | IL-17 inhibitors (CLE flare risk reported) [26,27,28,29,30,31]; TNF-α inhibitors [33,34,38,42,43]; hydroxychloroquine (psoriasis/PsA flares reported) [38,39] |
| Psoriatic arthritis + SLE | Mycophenolate mofetil [6,11]; methotrexate [6,11]; apremilast [16]; TYK2 inhibition (deucravacitinib) [35,36,37] | IL-23 inhibitors in stable SLE [12,13,14]; hydroxychloroquine with careful monitoring when psoriatic disease is quiescent [38,39] | TNF-α inhibitors [10,18,44,45,59]; hydroxychloroquine during active psoriasis/PsA [38,39]; phototherapy in SLE [9,20] |
| Subgroup | TNF-α Inhibitors | IL-17 Inhibitors | IL-23 Inhibitors | Ustekinumab (IL-12/23) | TYK2 Inhibitor | Apremilast | Methotrexate (MTX) | Mycophenolate (MMF) | Hydroxychloroquine (HCQ) | Phototherapy |
|---|---|---|---|---|---|---|---|---|---|---|
| Psoriasis + ANA+ (no lupus) | More frequently associated with ANA rise and DIL risk [10,18,22,23,24,25] | Generally associated with favorable safety profiles [47,49] | Generally associated with favorable safety profiles [12,13,14,32] | Used in selected contexts [33,46] | Limited overlap data; mechanistically favorable [35,36,37] | Generally well tolerated [16] | Used in selected contexts [6,11] | Infrequently required when SLE absent [6,11] | Use may require monitoring for psoriatic flares [38,39] | NB-UVB may be considered cautiously; higher-risk in ANA-high/ENA+ states [9,20,38] |
| Psoriasis + CLE | Frequently associated with CLE induction or exacerbation [10,18,33,34,38,42,43] | Frequently associated with CLE risk [26,27,28,29,30,31] | Generally associated with favorable safety profiles [12,13,14,32] | Neutral safety profile in limited data [33,34,46] | Emerging evidence suggests CLE benefit; limited PsO/PsA data [35,36,37] | Generally well tolerated [16] | Commonly used in overlap settings [6,11] | Commonly used in active CLE/SLE [6,11,20] | Requires caution due to psoriasis flare reports [38,39] | Frequently associated with photosensitive CLE risk [9,20] |
| Psoriasis + SLE | Frequently associated with lupus flares and DIL [10,18,34,39,43,44,45,50] | Frequently associated with CLE/lupus risk [26,27,28,29,30,31] | Used selectively in clinically stable SLE [12,13,14] | Reassuring safety with variable efficacy [33] | Mechanistically aligned with SLE/CLE biology; emerging evidence [35,36,37] | Generally well tolerated [16] | Commonly used in overlap disease [6,11] | Commonly used in overlap disease [6,11] | May be used selectively when psoriasis is mild and monitored [38,39] | Frequently associated with SLE photosensitivity risk [9,20,38] |
| PsA + ANA+ | Frequently associated with autoantibody induction and DIL risk [10,18,51,52,53,54] | Generally associated with favorable joint and serologic profiles [47,49] | Generally associated with favorable safety profiles [12,13,14,32] | Used in selected contexts [33,46] | Limited data; mechanistically favorable [35,36,37] | Used in selected contexts [16] | Commonly used for joint disease [6,11] | Used in selected contexts [6,11] | Requires caution if psoriatic disease active [38,39] | Use limited to low-titer ANA without lupus features [9,20] |
| PsA + CLE | Frequently associated with CLE risk [10,18,33,34,38,42,43] | Frequently associated with CLE risk [26,27,28,29,30,31] | Generally associated with favorable safety profiles [12,13,14,32] | Used in selected contexts [33,34,46] | CLE benefit reported; PsA data limited [35,36,37] | Used in selected contexts [16] | Commonly used for joint disease [6,11] | Commonly used in CLE/SLE-dominant overlap [6,11,20] | Frequently associated with psoriatic flares [38,39] | Frequently associated with CLE photosensitivity risk [9,20] |
| PsA + SLE | Frequently associated with lupus flares [10,18,44,45,59] | Frequently associated with CLE/lupus risk [26,27,28,29,30,31] | Used selectively in clinically stable SLE [12,13,14] | Used in selected contexts [33,34,46] | Dual IL-23/IFN-I modulation; emerging overlap data [35,36,37] | Generally well tolerated [16] | Commonly used for joint disease [6,11] | Commonly used in SLE-dominant disease [6,11] | Requires caution when PsA is active [38,39] | Frequently associated with SLE photosensitivity risk [9,20] |
| Immunologic Axis/Drug Class | Primary Mediators/Targets | Dominant Disease Context | Effect on Psoriasis/PsA | Effect on CLE/SLE | Interpretive Considerations in Overlap Disease |
|---|---|---|---|---|---|
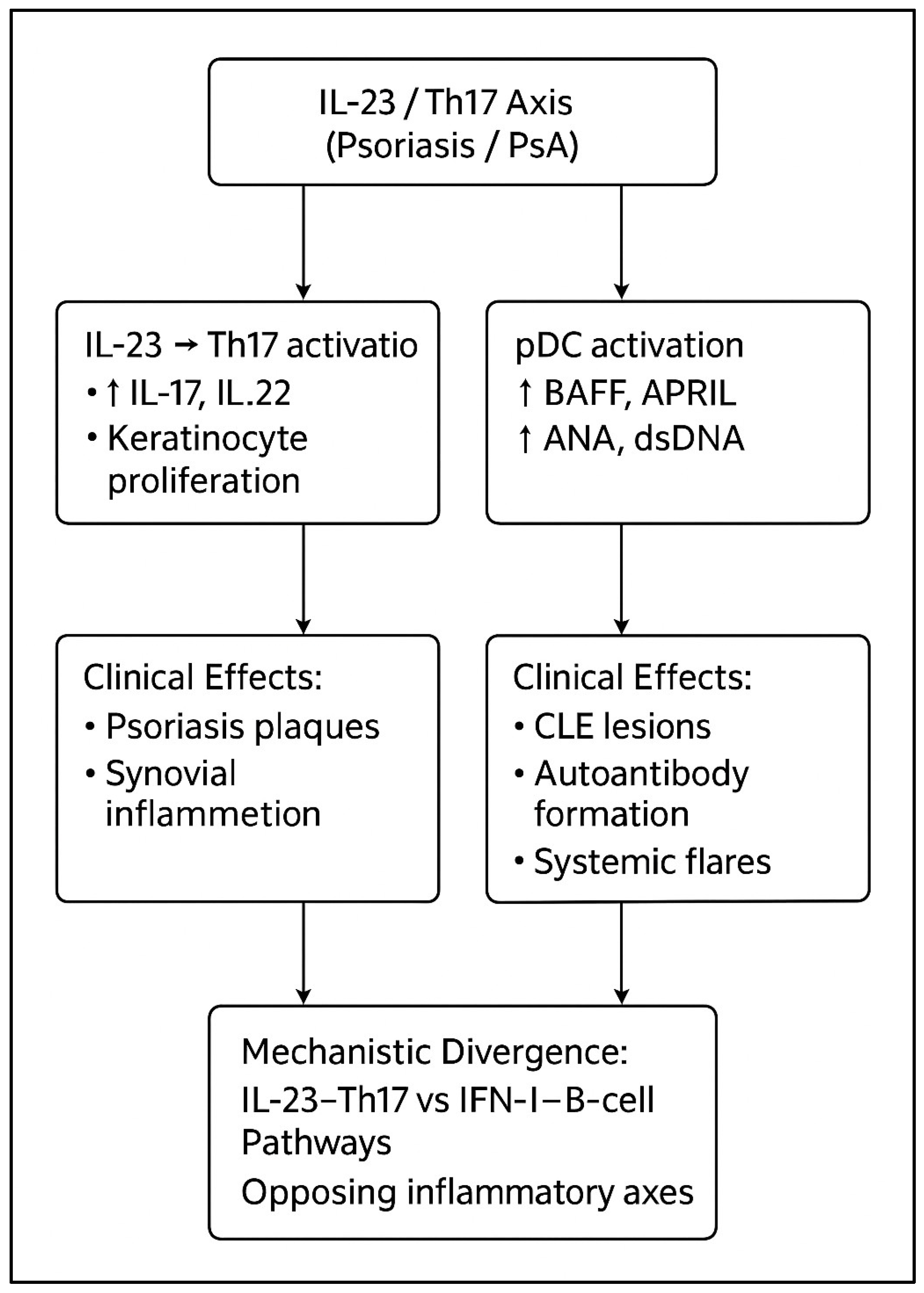
| Th17/IL-23 Axis | IL-23, IL-17A/F, IL-22, TNF-α | Psoriasis, PsA | Central to keratinocyte activation and synovial inflammation [1,2,3,4,5] | Indirect and generally secondary role [9,17] | Modulation of this axis is associated with improvement in psoriatic disease; downstream lupus-related effects appear to depend on accompanying changes in IFN-I activity [9,12,13,14,20] |
| Type I IFN/B-cell Axis | IFN-α/β, BAFF, ANA, dsDNA, immune complexes | CLE, SLE | May be secondarily activated but not a primary driver [6,7,8,9,17] | Central to cutaneous and systemic lupus activity [6,7,8,9,17] | Therapies that amplify IFN-I signaling or autoantibody production have been associated with increased lupus activity, whereas IFN-suppressive strategies are linked to more favorable lupus outcomes [9,17,20] |
| TNF-α inhibitors | TNF-α blockade | Psoriasis, PsA, RA | Highly effective for skin and joint disease [1,2,3,4,5] | Associated with ANA increase, dsDNA induction, drug-induced lupus, and CLE flares [10,18,22,23,24,25,33,34,38,42,43,44,45] | TNF-α inhibition is frequently associated with lupus-related serologic and clinical events in susceptible populations, which may warrant careful consideration in CLE/SLE-prone or high-risk ANA-positive patients [10,18,22,23,24,25,33,34,38,42,43,44,45] |
| IL-17 inhibitors | IL-17A/F blockade | Psoriasis, PsA | Very strong skin and joint efficacy [2,4,47,49] | Associated with de novo or exacerbated SCLE/DLE [26,27,28,29,30,31] | IL-17 blockade has been linked to cutaneous lupus manifestations in CLE-prone settings; use may require heightened vigilance in patients with active or high-risk cutaneous lupus phenotypes [26,27,28,29,30,31] |
| IL-23 inhibitors | IL-23 p19 blockade (upstream of Th17) | Psoriasis, PsA | Robust psoriatic disease control [12,13,14,32] | Neutral or potentially protective; no consistent lupus signal reported to date [12,13,14,32] | IL-23 inhibition has been associated with favorable cross-disease safety signals in available studies and may represent a relatively stable option across overlap phenotypes [12,13,14,32] |
| IL-12/23 inhibitor (ustekinumab) | p40 blockade | Psoriasis, PsA; studied in SLE | Effective in psoriasis and PsA [46] | Stable safety profile in SLE trials despite variable efficacy [33] | Ustekinumab is generally considered mechanistically neutral in overlap disease and may be used in selected contexts, particularly when IL-23-selective agents are unavailable [33,34,46] |
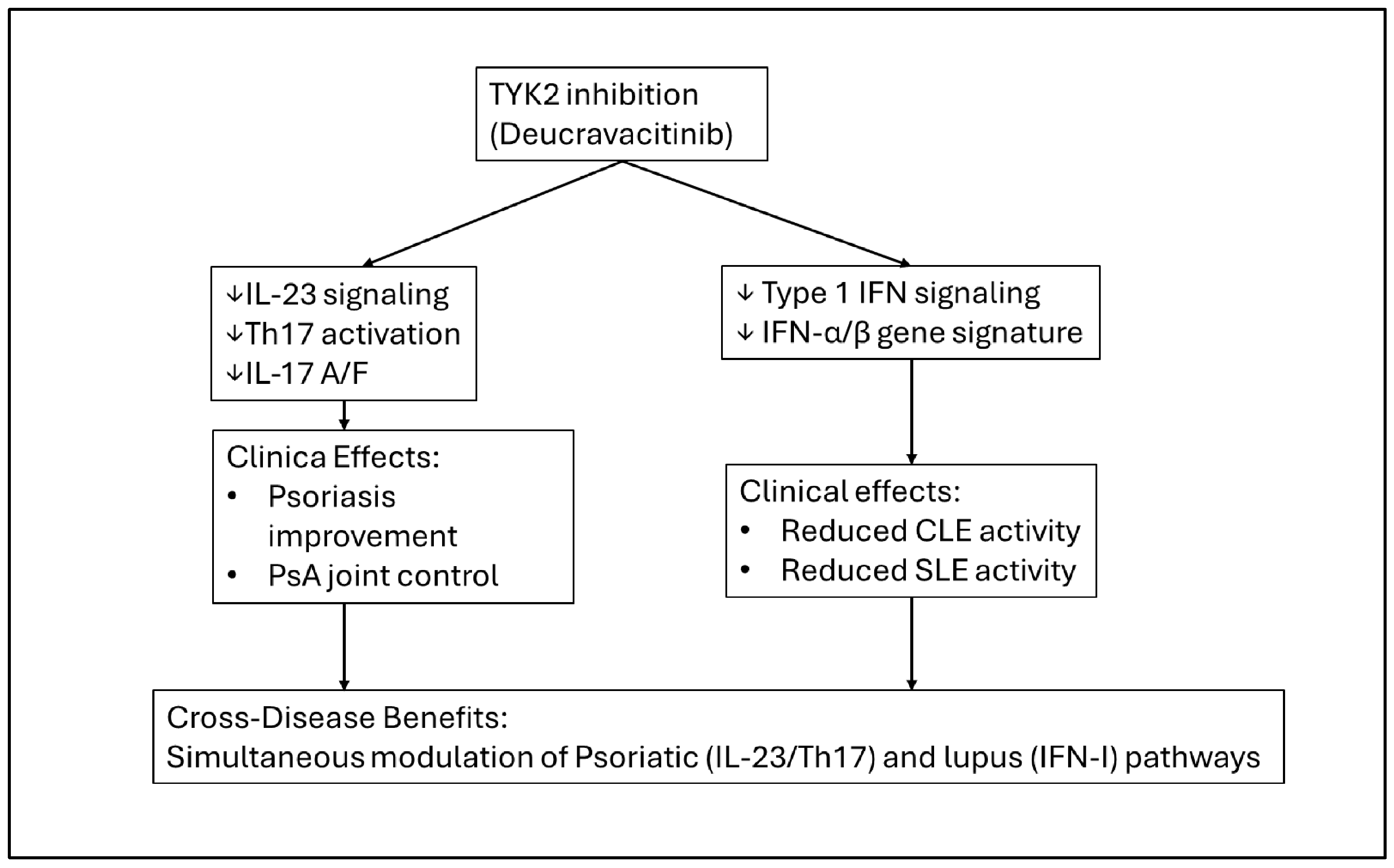
| TYK2 inhibitor (deucravacitinib) | TYK2 signaling (IL-23, IFN-I, IL-12 pathways) | Psoriasis; emerging data in SLE/CLE | Effective in psoriasis and PsA [35,36,37] | Suppression of IFN-I signatures and improvement in CLE/SLE endpoints reported [35,36,37] | Dual modulation of Th17 and IFN-I pathways suggests potential utility in overlap disease; evidence remains emerging [35,36,37] |
| PDE-4 inhibitor (apremilast) | cAMP-mediated cytokine modulation | Psoriasis, PsA | Moderate efficacy [16] | Neutral to mildly favorable effects reported in lupus [16] | Apremilast is generally well tolerated and may serve as an oral option in ANA-positive or lupus-prone patients [16] |
| Methotrexate (MTX) | Antimetabolite; T- and B-cell modulation | Psoriasis, PsA, SLE arthritis | Effective for joint and skin manifestations [6,11] | Beneficial for SLE musculoskeletal disease [6,11] | MTX is commonly used in SLE-dominant or mixed overlap phenotypes, particularly when inflammatory arthritis is prominent [6,11] |
| Mycophenolate mofetil (MMF) | Inhibition of lymphocyte proliferation | SLE, CLE | Modest effects on psoriasis/PsA [6,11] | Strong efficacy in SLE and CLE [6,11,20] | MMF is frequently employed in SLE- or CLE-dominant overlap presentations [6,11,20] |
| Hydroxychloroquine (HCQ) | TLR7/9 and IFN-I modulation | CLE, SLE | Psoriasis exacerbation reported in some patients [38,39] | Beneficial for CLE and SLE [6,11,38,39] | HCQ may be useful in lupus-dominant disease but requires careful risk–benefit assessment when psoriatic disease is active [38,39] |
| Phototherapy (NB-UVB/UVB) | UV-induced keratinocyte apoptosis and neo-antigen exposure | Psoriasis | Effective for psoriatic skin disease [63] | CLE flares reported via IFN-I upregulation [9,20] | Phototherapy has been associated with photosensitive lupus activity and may be more suitable in low-risk ANA-positive patients without CLE/SLE features [9,20,38] |
| Therapeutic Class | Primary Drug-Associated Autoimmune Signal(s) | Strength of Evidence | Typical Clinical Phenotype | Reversibility After Drug Withdrawal | Overall Pattern of Drug-Induced Autoimmunity (Revised) |
|---|---|---|---|---|---|
| TNF-α inhibitors | ANA seroconversion; anti-dsDNA induction; anti-TNF-induced lupus (ATIL); CLE-like eruptions [10,18,22,23,24,25,33,34,38,42,43,44,45] | High—multiple cohorts, case series, pharmacovigilance data [10,18,22,23,24,25,33,34,38,42,43] | Photosensitive rash; SCLE/DLE-like lesions; arthritis/serositis; ANA ± dsDNA; occasional systemic lupus features [33,34,38,42,43,44,45] | Typically improves or resolves after withdrawal ± corticosteroids or hydroxychloroquine [33,34,38,42,43] | Frequently associated with lupus-related serologic and clinical events in susceptible populations; careful risk–benefit consideration is often warranted in CLE- or SLE-prone patients [10,18,22,23,24,25,33,34,38,42,43,44,45] |
| IL-17 inhibitors | New-onset or exacerbated CLE (SCLE/DLE); rare lupus-like events [26,27,28,29,30,31] | Moderate—increasing case reports and series [26,27,28,29,30,31] | Disseminated DLE/SCLE; photo-exacerbated plaques; ANA elevation with minimal systemic involvement [26,27,28,29,30,31] | Improvement commonly reported after drug withdrawal; switching to non-Th17 agents described [26,27,28,29,30,31] | Cutaneous lupus manifestations reported with greater frequency in CLE-prone settings; heightened vigilance may be appropriate in active DLE/SCLE [26,27,28,29,30,31] |
| IL-23 inhibitors | Occasional ANA changes; no consistent lupus/CLE signal [12,13,14,32] | Low—pooled trial data and real-world reports [12,13,14,32] | Isolated autoantibody changes; lupus events rare and not clearly drug-related [12,13,14] | Withdrawal typically not required | Generally associated with reassuring lupus-related safety signals across reported overlap phenotypes [12,13,14,32] |
| IL-12/23 inhibitor (ustekinumab) | Rare lupus-like or autoimmune phenomena; neutral findings in SLE trials [33,46] | Low–moderate—Phase II/III SLE and psoriasis data [33,46] | Stable SLE activity; no consistent CLE or systemic flares reported [33] | Withdrawal rarely required [33] | Mechanistically neutral safety profile in available data; commonly considered in psoriasis with stable SLE [33,34,46] |
| TYK2 inhibitor (deucravacitinib) | Reduction in IFN-driven autoimmunity; improvement in CLE/SLE markers reported [35,36,37] | Emerging—early SLE/CLE trials and transcriptomic studies [35,36,37] | Decreased IFN signatures; improved CLE/SLE activity measures; no de novo lupus reported [35,36,37] | Not typically associated with drug-induced autoimmunity | Emerging evidence suggests a favorable immunologic profile in overlap disease; data remain limited [35,36,37] |
| PDE-4 inhibitor (apremilast) | Minimal autoimmunity signal; rare nonspecific immune events [16] | Low—extensive psoriasis/PsA use; few lupus reports [16] | Mild, nonspecific immune findings; no consistent ANA/CLE/SLE pattern [16] | Generally reversible; therapy often continued | Generally well tolerated in ANA-positive and lupus-prone populations in reported studies [16] |
| Methotrexate (MTX) | No drug-induced lupus signature; reduction in SLE activity reported [6,11] | Low—long clinical experience [6,11] | Improvement in joint, skin, and systemic inflammatory manifestations [6,11] | Not applicable (not lupus-inducing) | Widely used with a stable autoimmune safety profile, particularly in SLE-dominant overlap phenotypes [6,11] |
| Mycophenolate mofetil (MMF) | Treatment of SLE/CLE; no drug-induced lupus reported [6,11,20] | Low—standard SLE/CLE therapy [6,11] | Reduction in CLE lesions and SLE activity [6,11,20] | Not applicable (therapeutic rather than inductive) | Commonly employed in SLE/CLE-dominant overlap disease with protective lupus effects [6,11,20] |
| Hydroxychloroquine (HCQ) | Psoriasis flares; paradoxical psoriatic autoimmunity reported [38,39] | Moderate—multiple psoriasis flare reports [38,39] | New-onset psoriasis or worsening of existing disease; lupus control generally maintained [38,39] | Psoriasis often improves after withdrawal [38,39] | Low lupus risk but notable psoriasis flare risk; individualized assessment needed when psoriatic disease is active [38,39] |
| Rituximab | Improvement in SLE; paradoxical psoriasis induction reported [40] | Moderate—case reports and series [40] | De novo psoriasis or flares; concurrent SLE improvement [40] | Psoriasis often improves after withdrawal [40] | Low lupus-induction risk but psoriasis exacerbation reported; relevance depends on psoriatic disease activity [40] |
| Phototherapy (NB-UVB/UVB) | Photo-induced CLE/DLE/SCLE; IFN-signature amplification [9,20,38] | Moderate—well-documented UV-triggered CLE [9,20] | New or worsening CLE in sun-exposed areas; systemic flares less common [9,20,38] | Improvement reported after cessation and photoprotection [9,20] | Cutaneous lupus flares reported in photosensitive or ANA-high individuals; risk varies by phenotype and exposure [9,20,38] |
| Agent | Study/Population | Design and Sample | Key Efficacy Findings in SLE/CLE | Safety Signals | Interpretive Considerations for Psoriasis/PsA with Lupus-Spectrum Disease |
|---|---|---|---|---|---|
| Ustekinumab (IL-12/23 inhibitor) | Phase II SLE trial [33] | Randomized, placebo-controlled; moderate-to-severe SLE | Improved SRI-4 responses compared with placebo; benefits observed in musculoskeletal and mucocutaneous domains [33] | No increase in lupus flares; no drug-induced lupus (DIL); no new safety concerns reported [33] | Demonstrates biologic activity in SLE with a stable safety profile; findings suggest potential suitability in psoriasis/PsA patients with stable or mild SLE, particularly when IL-23-selective agents are unavailable [33,34,46] |
| Phase III SLE trial [33] | Randomized, placebo-controlled; multicenter SLE cohort | Did not meet the primary BICLA endpoint; modest improvements observed in selected secondary outcomes (e.g., SRI-6) [33] | Safety profile comparable to placebo; no lupus activation reported [33] | Indicates stable safety with variable SLE efficacy; supports consideration of ustekinumab as a mechanistically neutral option in overlap disease rather than as a primary lupus-directed therapy [33,34] | |
| Case reports/small CLE series [33,34] | SCLE/DLE patients treated off-label | Variable CLE lesion responses, with improvement reported in some refractory cases [33,34] | No CLE worsening or systemic lupus induction reported [33] | Suggests ustekinumab is unlikely to exacerbate CLE in psoriatic patients, although evidence is limited and non-randomized [33,34] | |
| Deucravacitinib (TYK2 inhibitor) | Phase II SLE trial [35,36,37] | Randomized, placebo-controlled; active SLE with multi-organ involvement | Improvements reported in patient-reported outcomes and global disease activity; reductions in IFN-I-driven gene signatures observed [35,36,37] | No DIL signal; no major lupus flares reported; generally well tolerated [35,36,37] | Provides proof-of-concept that TYK2 targeting may modulate SLE activity while influencing IFN-I pathways, which is mechanistically relevant to overlap disease [35,36,37] |
| CLE transcriptomic study [36] | CLE patients; mechanistic/transcriptomic analysis | Decreased IFN-I-regulated transcripts and reduced CLE inflammatory signatures observed [36] | No new safety concerns reported [36] | Indicates potential direct effects on CLE-associated molecular pathways, supporting further exploration in overlap settings [36] | |
| Real-world PsO + PsA + SLE case [35] | Single-patient refractory overlap treated with deucravacitinib plus MMF and HCQ | Concurrent improvement in psoriasis, PsA, and SLE with steroid-sparing effect reported [35] | No lupus activation or psoriasis flare reported [35] | Illustrates feasibility of TYK2 inhibition in complex overlap disease; interpretation limited by single-case design [35] | |
| Psoriasis Phase III trials (contextual) [35,36,37] | Phase III randomized controlled trials in psoriasis | High PASI-75/90 response rates and durable efficacy reported [35,36,37] | No CLE or SLE signals reported in psoriasis trials [35,36,37] | Confirms strong psoriatic efficacy with no evident lupus-related safety signals in trial populations; relevance to overlap disease remains to be defined in dedicated studies [35,36,37] |
| Clinical Setting | Phototherapy Modality | Psoriasis/PsA Efficacy | Lupus/Autoimmunity Considerations | Context-Dependent Use Considerations |
|---|---|---|---|---|
| Psoriasis/PsA, ANA-negative | NB-UVB, BB-UVB | High efficacy for plaque psoriasis; useful for PsA with skin-dominant disease [63] | No lupus-specific safety signals reported | Phototherapy is commonly used in the absence of lupus features or autoimmune history |
| Psoriasis/PsA, low-titer ANA positivity (no ENA, no lupus symptoms) | NB-UVB preferred; avoidance of high cumulative doses | Effective psoriasis control; may reduce need for systemic therapy [63] | Theoretical IFN-I upregulation and autoantibody evolution reported, but clinical risk appears low in available studies [9,20] | NB-UVB may be considered with exposure limitation and clinical monitoring for CLE-like changes or systemic symptoms [9,20] |
| Psoriasis/PsA, high-titer ANA or ENA positivity (no overt lupus) | NB-UVB only in selected cases; avoidance of PUVA or high-dose UVB | Psoriatic improvement reported, though alternative systemic options are often available [6,11] | Increased susceptibility to UV-provoked autoimmunity and CLE-like changes reported; IFN-I pathway activation described [9,20,38] | Use may be reserved for situations where systemic therapies are unsuitable, with short treatment courses and close dermatologic and serologic monitoring [9,20,38] |
| Psoriasis with CLE (SCLE or DLE) | NB-UVB, BB-UVB, PUVA | Psoriasis may improve; CLE lesions are typically photosensitive [9,20,38] | CLE flares, new lesion development, and IFN-I amplification frequently reported [9,20] | Phototherapy has been associated with higher cutaneous lupus risk; systemic or biologic alternatives with IFN-compatible profiles are often considered, along with strict photoprotection [6,11,12,13,14,16] |
| PsA with CLE | NB-UVB, BB-UVB, PUVA | Limited data; some improvement in psoriatic manifestations reported | Similar risk of CLE exacerbation as psoriasis with CLE [9,20,38] | Phototherapy has been associated with CLE worsening; management often prioritizes systemic agents active across joint and cutaneous disease [6,11,12,13,14] |
| Psoriasis/PsA with SLE (±CLE) | NB-UVB, BB-UVB, PUVA | Psoriatic skin disease may improve; no benefit for systemic lupus activity [63] | Photosensitive SLE and CLE flares well documented; UV exposure is a recognized trigger [9,20,38] | Phototherapy has been associated with lupus activation in many reports; alternative non-UV systemic strategies and rigorous photoprotection are typically emphasized [9,20,38] |
| Isolated CLE or SLE without psoriasis/PsA | Any UV-based modality | Not indicated for lupus management [6,11] | High frequency of cutaneous and systemic lupus flares reported with UV exposure [9,20,38] | UV-based therapies are generally not used in lupus-only disease; management relies on systemic immunomodulatory therapies compatible with IFN-mediated biology [6,11,20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Tjiu, J.-W.; Tsai, T.-F. Systemic Treatment Strategies for Patients with Psoriasis and Psoriatic Arthritis in the Setting of ANA Positivity or Lupus Spectrum Disease: A Comprehensive Systematic Review. Int. J. Mol. Sci. 2026, 27, 1093. https://doi.org/10.3390/ijms27021093
Tjiu J-W, Tsai T-F. Systemic Treatment Strategies for Patients with Psoriasis and Psoriatic Arthritis in the Setting of ANA Positivity or Lupus Spectrum Disease: A Comprehensive Systematic Review. International Journal of Molecular Sciences. 2026; 27(2):1093. https://doi.org/10.3390/ijms27021093
Chicago/Turabian StyleTjiu, Jeng-Wei, and Tsen-Fang Tsai. 2026. "Systemic Treatment Strategies for Patients with Psoriasis and Psoriatic Arthritis in the Setting of ANA Positivity or Lupus Spectrum Disease: A Comprehensive Systematic Review" International Journal of Molecular Sciences 27, no. 2: 1093. https://doi.org/10.3390/ijms27021093
APA StyleTjiu, J.-W., & Tsai, T.-F. (2026). Systemic Treatment Strategies for Patients with Psoriasis and Psoriatic Arthritis in the Setting of ANA Positivity or Lupus Spectrum Disease: A Comprehensive Systematic Review. International Journal of Molecular Sciences, 27(2), 1093. https://doi.org/10.3390/ijms27021093







