Coacervated and Freeze-Dried Polysaccharides-Nanoparticle with Efficient Encapsulation of Albendazole for High-Performance Treatment of Monogenean Parasite Infestation in Tilapia Fish
Abstract
1. Introduction
2. Results and Discussion
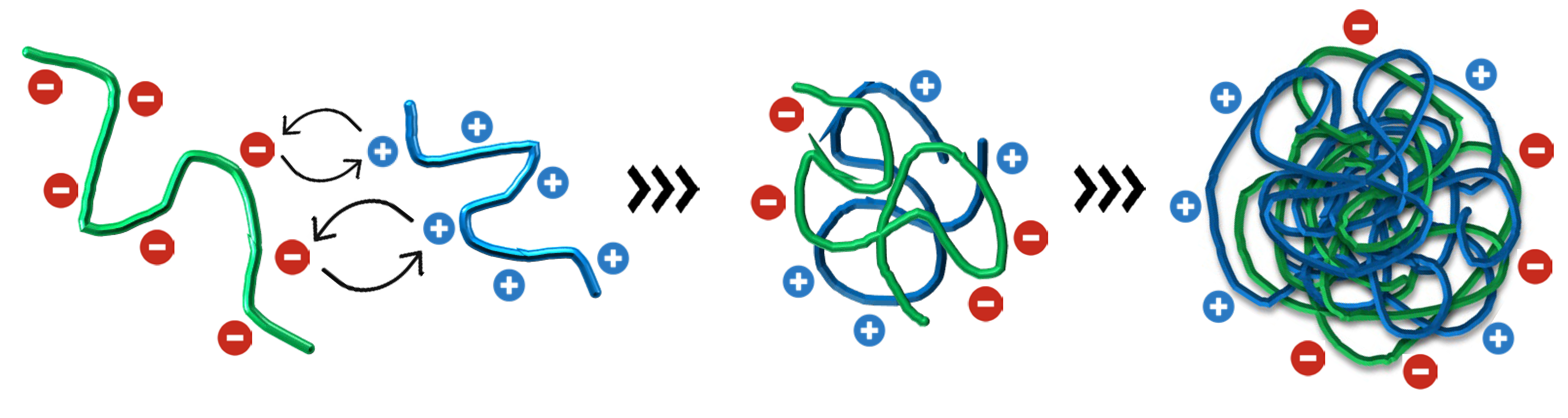
2.1. Thermodynamics of Nanoparticle Production
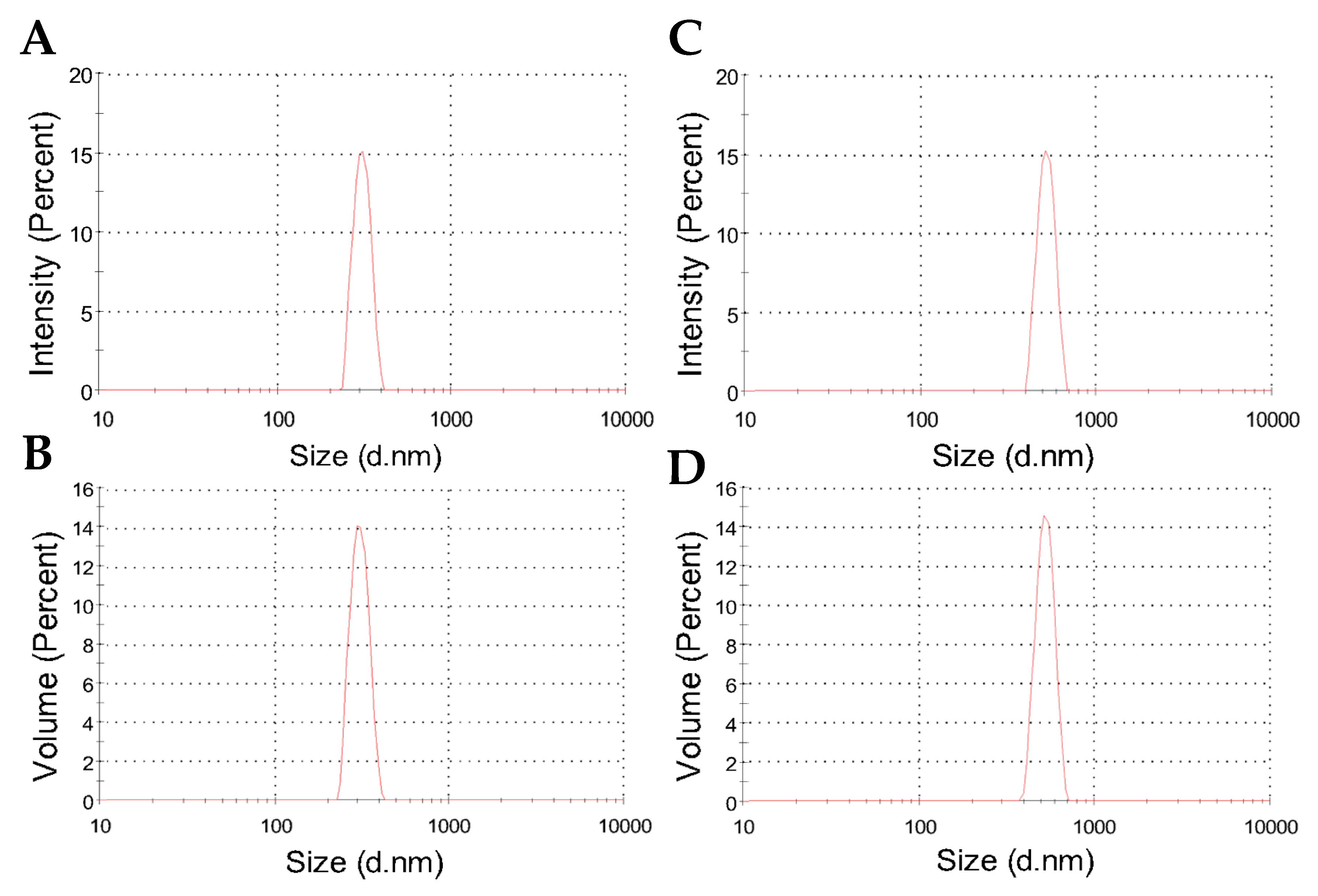
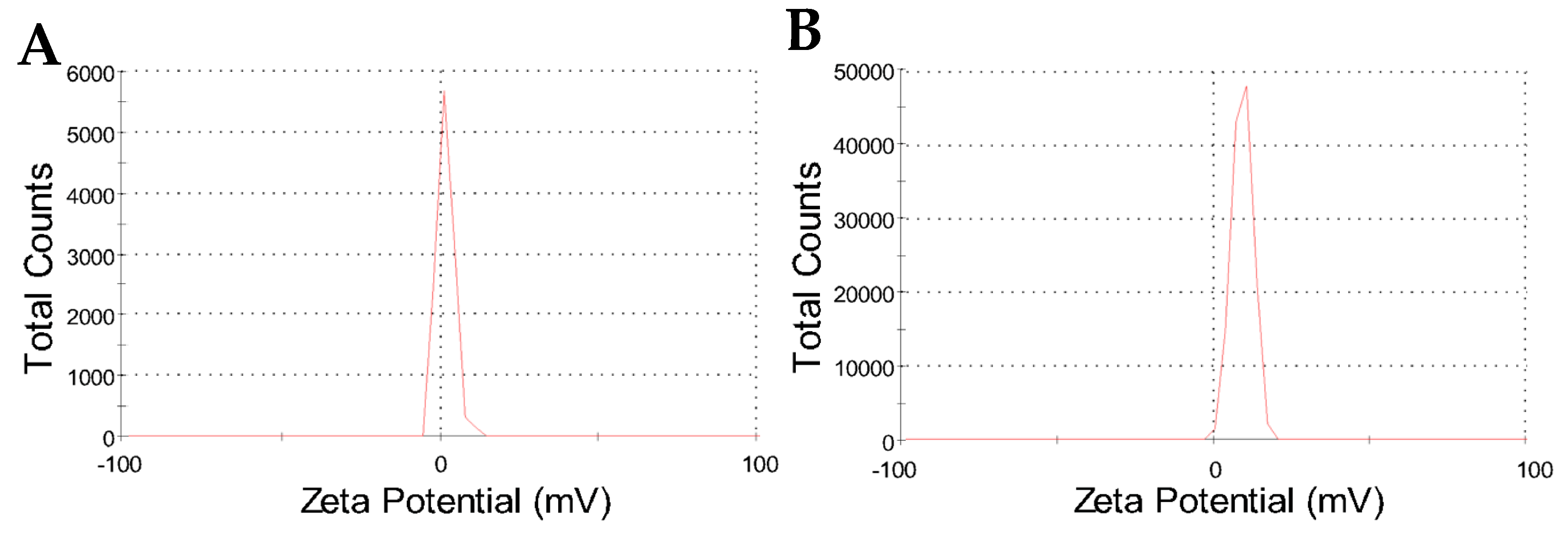
2.2. Colloidal Features of the Nanoparticles
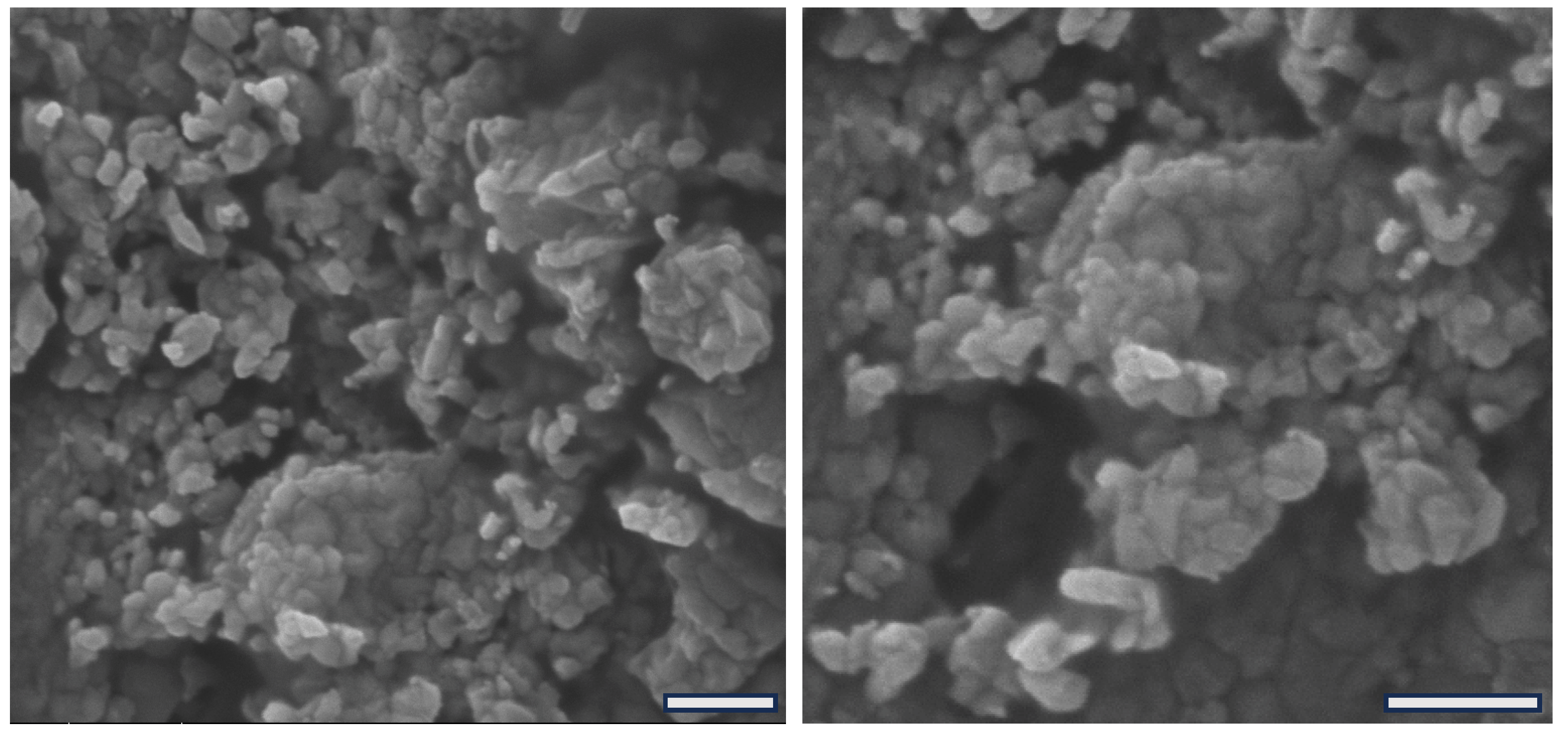
2.3. Scanning Electron Microscopy of Freeze-Dried Nanoparticles
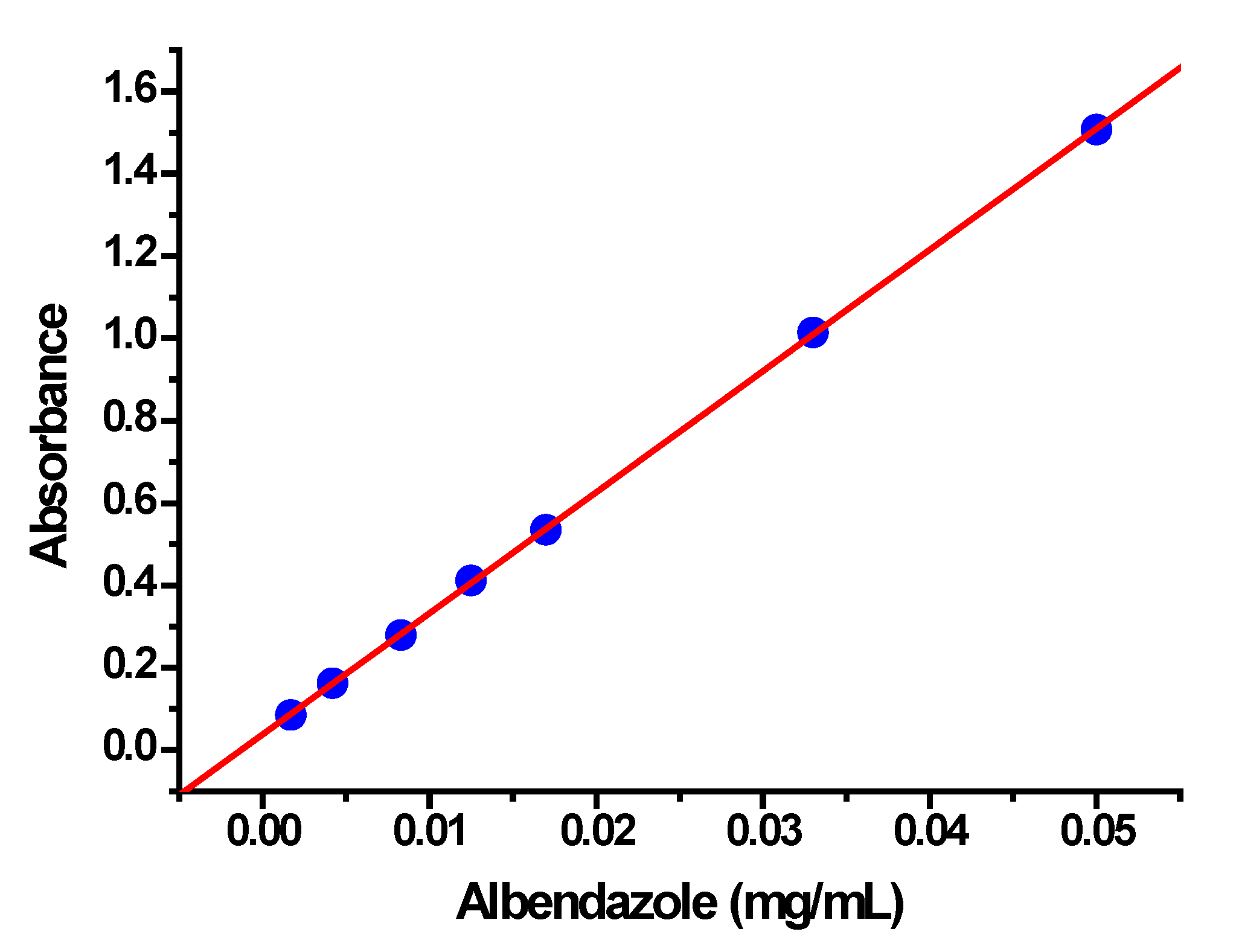
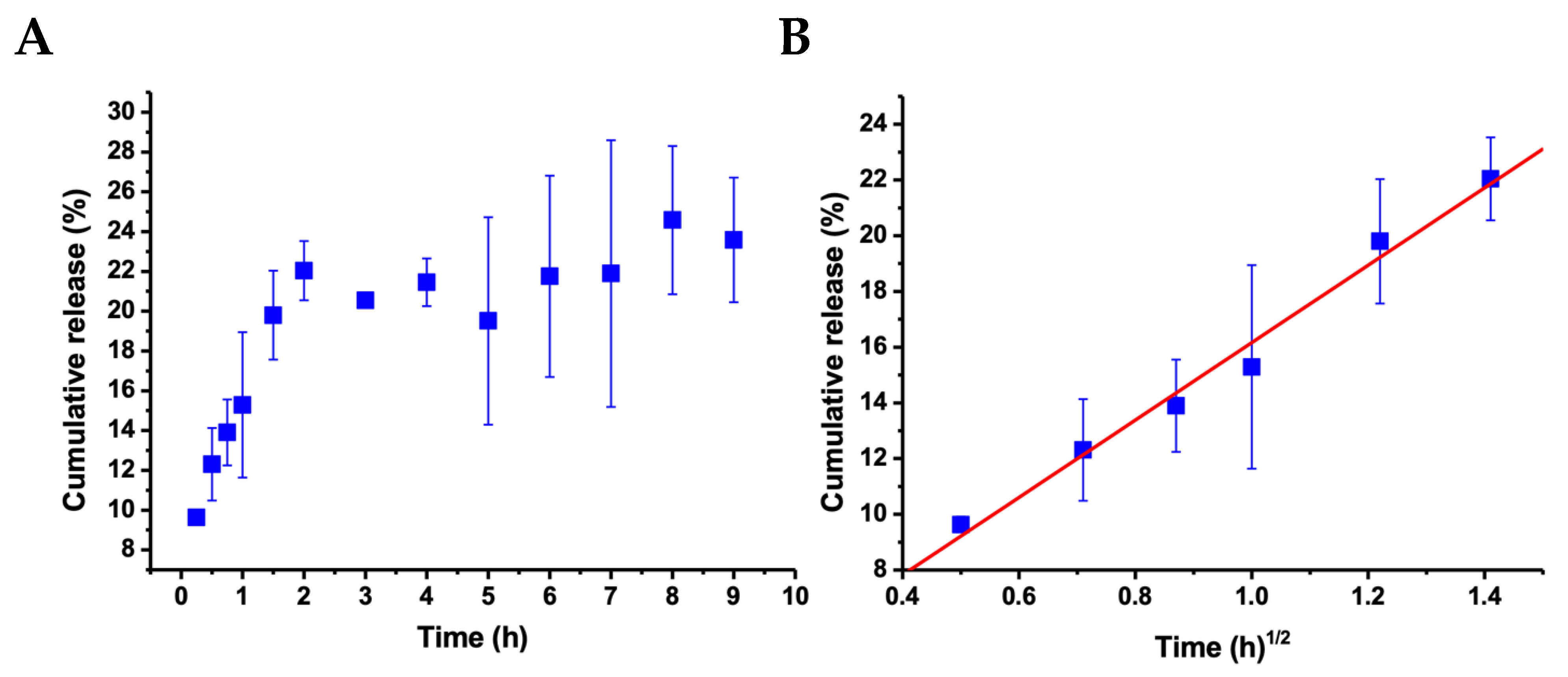
2.4. Encapsulation and Release of Albendazole
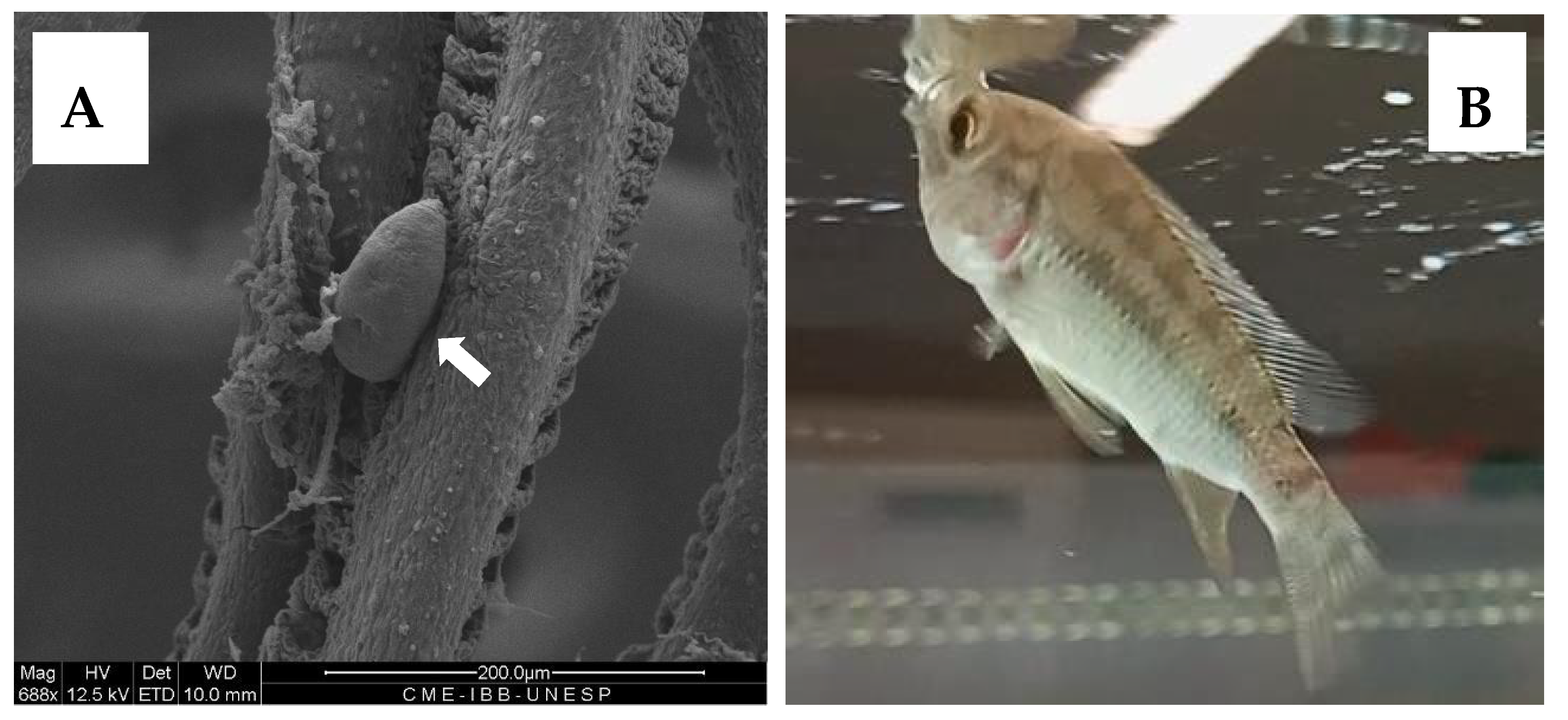
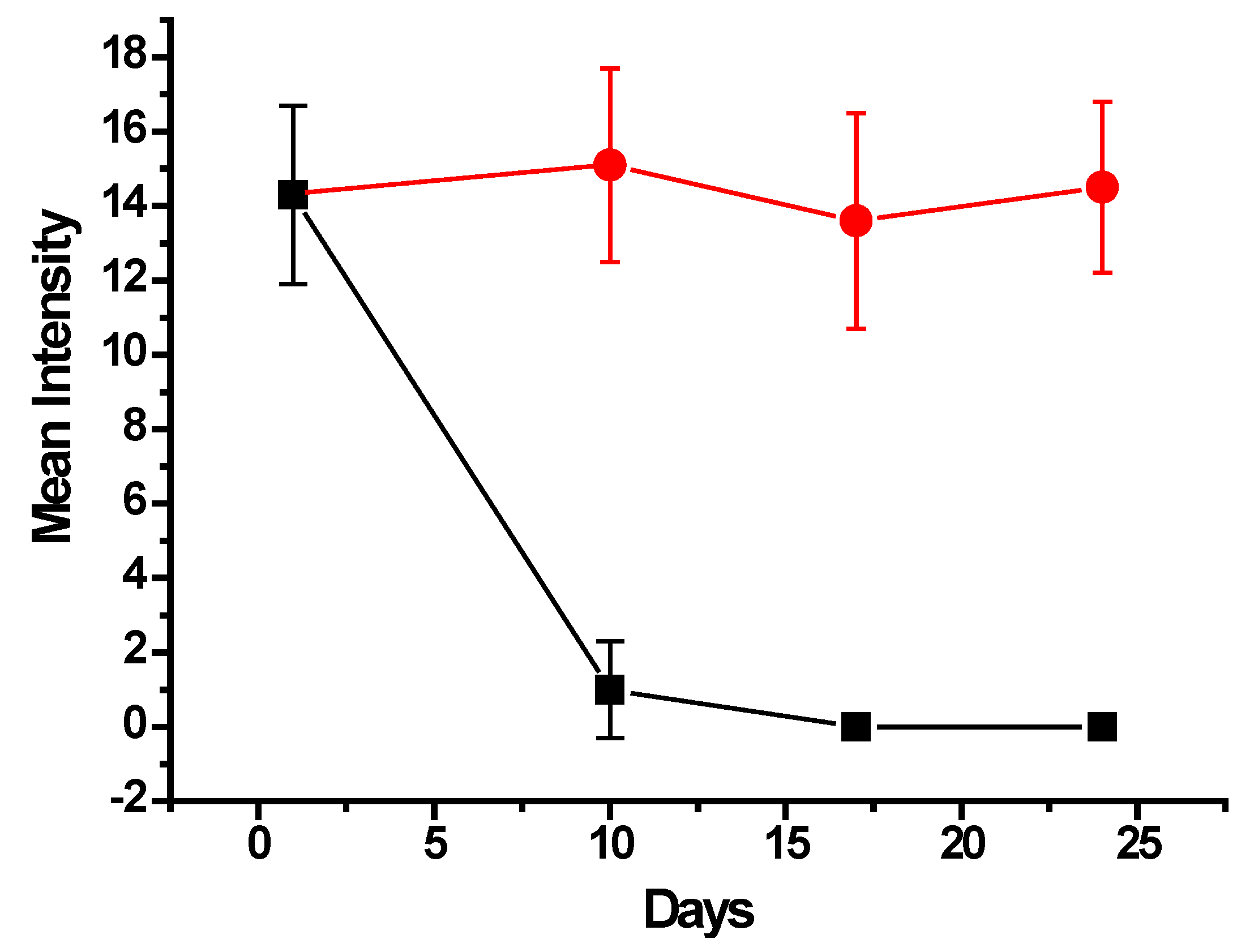
2.5. In Vivo Application and Parasite Treatment
3. Materials and Methods
3.1. Materials
3.2. Nanoparticles Preparation
3.3. Isothermal Titration Calorimetry (ITC)
3.4. Dynamic Light Scattering and Zeta Potential
3.5. Scanning Electron Microscopy (SEM)
3.6. Encapsulation and Release of Albendazole
3.7. In Vivo Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talarek, E.; Aniszewska, M.; Dobrzeniecka, A.; Kmiotek, J.; Pokorska-Śpiewak, M. Autoimmune Hepatitis After Successful Treatment of Chronic Hepatitis C Virus Infection with Direct-Acting Antivirals: A Pediatric Case Report. Pathogens 2025, 14, 1244. [Google Scholar] [CrossRef]
- Graeber, S.Y.; Mall, M.A. The future of cystic fibrosis treatment: From disease mechanisms to novel therapeutic approaches. Lancet 2023, 402, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Agbosu, E.E.; Ledger, S.; Kelleher, A.D.; Wen, J.; Ahlenstiel, C.L. Targeted Nanocarrier Delivery of RNA Therapeutics to Control HIV Infection. Pharmaceutics 2022, 14, 1352. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.I.; Ahammad, F.; Mohammed, H. Cutting-edge technologies for detecting and controlling fish diseases: Current status, outlook, and challenges. J. World Aquac. Soc. 2024, 55, e13051. [Google Scholar] [CrossRef]
- Batista, D.V.V.; Jatobá, A.; da Silva, A.V.; de Souza, A.P.; Farias, C.F.S.; Lopes, E.M.; Owatari, M.S.; Ventura, A.S.; Cardoso, C.A.L.; Fontes, S.T.; et al. Therapeutic Efficacy of Monoterpenes in Nile Tilapia Infected with Edwardsiella tarda: A Phytogenic Alternative to Oxytetracycline. J. Fish Dis. 2025, 49, e70032. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.; Trataris-Rebisz, A.N.; Tempia, S.; Rostal, M.K.; Karesh, W.B.; Msimang, V. Seroprevalence and Associated Risk Factors of Human Brucellosis in a Farming and Animal Health Community in South Africa, 2015–2016. Trop. Med. Infect. Dis. 2025, 10, 302. [Google Scholar] [CrossRef]
- Bellet, C.; Hamilton, L.; Rushton, J. Re-thinking public health: Towards a new scientific logic of routine animal health care in European industrial farming. Humanit. Soc. Sci. Commun. 2021, 8, 214. [Google Scholar] [CrossRef]
- Mazac, R.; Sahlin, K.R.; Hyypiä, I.; Keränen, F.; Niva, N.; Berglund, N.; Herzon, I. Does “better” mean “less”? Sustainable meat consumption in the context of natural pasture-raised beef. Agric. Hum. Values 2025, 42, 1637–1651. [Google Scholar] [CrossRef]
- Deb, P.; Dey, M.M.; Surathkal, P. Price transmission and market integration of Bangladesh fish markets. Aquaculture 2022, 560, 738592. [Google Scholar] [CrossRef]
- Seibel, H.; Weirup, L.; Schulz, C. Fish Welfare—Between Regulations, Scientific Facts and Human Perception. Food Ethics 2020, 5, 4. [Google Scholar] [CrossRef]
- Röcklinsberg, H. Fish Consumption: Choices in the Intersection of Public Concern, Fish Welfare, Food Security, Human Health and Climate Change. J. Agric. Environ. Ethics 2015, 28, 533–551. [Google Scholar] [CrossRef]
- Ziarati, M.; Zorriehzahra, M.J.; Hassantabar, F.; Mehrabi, Z.; Dhawan, M.; Sharun, K.; Bin Emran, T.; Dhama, K.; Chaicumpa, W.; Shamsi, S. Zoonotic diseases of fish and their prevention and control. Vet. Quarterly 2022, 42, 95–118. [Google Scholar] [CrossRef]
- Novoslavskij, A.; Terentjeva, M.; Eizenberga, I.; Valcina, O.; Bartkevics, V.; Berzins, A. Major foodborne pathogens in fish and fish products: A review. Ann. Microbiol. 2016, 66, 1–15. [Google Scholar] [CrossRef]
- Shinn, A.J.; Pratoomyot, J.; Bron, J.; Paladini, G.; Brooker, E.; Brooker, A. Economic impacts of aquatic parasites on global finfish production. Glob. Aquacul. Advocate 2015, 82–84. [Google Scholar]
- Maezono, M.; Nielsen, R.; Buchmann, K.; Nielsen, M. The Current State of Knowledge of the Economic Impact of Diseases in Global Aquaculture. Rev. Aquac. 2025, 17, e70039. [Google Scholar] [CrossRef]
- Tavares-Dias, M.; Martins, M.L. An overall estimation of losses caused by diseases in the Brazilian fish farms. J. Parasit. Dis. 2017, 41, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Hoai, T.D. Reproductive strategies of parasitic flatworms (Platyhelminthes, Monogenea): The impact on parasite management in aquaculture. Aquacult. Int. 2020, 28, 421–447. [Google Scholar] [CrossRef]
- Mathews, P.D.; Mertins, O.; Mathews, J.P.D.; Ismiño, O.R. Massive parasitism by Gussevia tucunarense (Platyhelminthes: Monogenea: Dactylogyridae) in fingerlings of bujurqui-tucunare cultured in the Peruvian Amazon. Acta Parasitol. 2013, 58, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, A.F.; Mathews, P.D.; Luna, L.E.; Mathews, J.D. Outbreak of Notozothecium bethae (Monogenea: Dactylogyridae) in Myleus schomburgkii (Actinopterygii: Characiformes) cultured in the Peruvian Amazon. J. Parasit. Dis. 2016, 40, 1631–1635. [Google Scholar] [CrossRef]
- Alves, C.M.G.; Nogueira, J.N.; Barriga, I.B.; Santos, J.R.; Santos, G.G.; Tavares-Dias, M. Albendazole, levamisole and ivermectin are effective against monogeneans of Colossoma macropomum (Pisces: Serrasalmidae). J. Fish Dis. 2019, 42, 405–412. [Google Scholar] [CrossRef]
- Kang, Y.J.; Kim, D.S.; Kim, K.H. Evaluation of treatment efficacy of doxycycline and albendazole against scuticociliatosis in olive flounder (Paralichthys olivaceus). Aquaculture 2013, 416, 192–195. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, Y.; Zhang, Y.-N.; Wang, W.-R.; Chen, Y.-X.; Jin, Y.-G.; Sun, L.-J.; Li, S.-H.; Yang, F.; Li, X.-P.; et al. Depletion of Albendazole and Its Metabolites and Their Impact on the Gut Microbial Community Following Multiple Oral Dosing in Yellow River Carp (Cyprinus carpio haematopterus). Fishes 2025, 10, 410. [Google Scholar] [CrossRef]
- Shaikh, B.; Rummel, N.; Gieseker, C.; Cheely, C.S.; Reimschuessel, R. Residue depletion of albendazole and its metabolites in the muscle tissue of large mouth and hybrid striped bass after oral administration. J. Chromatogr. A 2009, 1216, 8173–8176. [Google Scholar] [CrossRef]
- Negreiros, L.P.; Souza, E.X.; Lima, T.A.; Tavares-Dias, M. Albendazole is effective for controlling monogenean parasites of the gills of Piaractus brachypomus (Serrasalmidae) and Megaleporinus macrocephalus (Anostomidae). Braz. J. Vet. Parasitol. 2022, 31, e010322. [Google Scholar] [CrossRef] [PubMed]
- Farias, C.F.S.; Brandão, F.R.; Sebastião, F.A.; Souza, C.M.; Monteiro, P.C.; Majolo, C.; Chagas, E.C. Albendazole and praziquantel for the control of Neoechinorhynchus buttnerae in tambaqui (Colossoma macropomum). Aquacult. Int. 2021, 29, 1495–1505. [Google Scholar] [CrossRef]
- Bunnnajirakul, S.; Phalitakul, S.; Sanisuriwong, J. Therapeutic effect of albendazole and praziquantel for digenean trematode (metacercarial stage) treatment in goldfish (Carassius auratus). J. Mahanakorn Vet. Med. 2009, 4, 46–55. [Google Scholar]
- Shaikh, B.; Rummel, N.; Gieseker, C.; Serfling, S.; Reimschuessel, R. Metabolism and residue depletion of albendazole and its metabolites in rainbow trout, tilapia and Atlantic salmon after oral administration. J. Vet. Pharm. Therap. 2003, 26, 421–427. [Google Scholar] [CrossRef]
- Cordeiro, R.P.; Braga, P.A.C.; Jonsson, C.M.; Brandao, F.R.; Chagas, E.C.; Reyes, F.G.R. Therapeutic efficacy and bioaccumulation of albendazole in the treatment of tambaqui (Colossoma macropomum) parasitized by acanthocephalan (Neoechinorhynchus buttnerae). Aquacult. Res. 2022, 53, 1446–1455. [Google Scholar] [CrossRef]
- Dai, Y.; Yang, H.-Y.; Yang, F.; Li, X.; Liu, Y.; Jin, Y.-G.; Li, Z.-E.; Duan, M.-H.; Zhang, Y.-N. Pharmacokinetics and Tissue Distribution of Albendazole and Its Three Metabolites in Yellow River Carp (Cyprinus carpio haematopterus) after Single Oral Administration. J. Agric. Food Chem. 2025, 73, 1824–1834. [Google Scholar] [CrossRef]
- Yu, D.; Evans, E.R.; Hasbrouck, N.; Reimschuessel, R.; Shaikh, B. Residue depletion of albendazole and its metabolites in aquacultured yellow perch (Perca flavescens). J. Vet. Pharmacol. Therap. 2012, 35, 560–562. [Google Scholar] [CrossRef]
- Oxendine, S.L.; Cowden, J.; Hinton, D.E.; Padilla, S. Vulnerable windows for developmental ethanol toxicity in the Japanese medaka fish (Oryzias latipes). Aquatic Toxicol. 2006, 80, 396–404. [Google Scholar] [CrossRef]
- Kaviraj, A.; Bhunia, F.; Saha, N.C. Toxicity of Methanol to Fish, Crustacean, Oligochaete Worm, and Aquatic Ecosystem. Int. J. Toxicol. 2004, 23, 55–63. [Google Scholar] [CrossRef]
- Andrade-Vieira, L.F.; Bojic, C.; Santana Alvarenga, I.F.; de Carvalho, T.S.; Masfaraud, J.F.; Cotelle, S. Ecotoxic effects of the vehicle solvent dimethyl sulfoxide on Raphidocelis subcapitata, Daphnia magna and Brachionus calyciflorus. Chem. Ecol. 2022, 38, 471–483. [Google Scholar] [CrossRef]
- Kong, L.; Campbell, F.; Kros, A. DePEGylation strategies to increase cancer nanomedicine efficacy. Nanoscale Horiz. 2019, 4, 378–387. [Google Scholar] [CrossRef]
- Salamanna, F.; Gambardella, A.; Contartese, D.; Visani, A.; Fini, M. Nano-Based Biomaterials as Drug Delivery Systems Against Osteoporosis: A Systematic Review of Preclinical and Clinical Evidence. Nanomaterials 2021, 112, 530. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Dominguez, D.J.; López-Enríquez, S.; Alba, G.; Garnacho, C.; Jiménez-Cortegana, C.; Flores-Campos, R.; de la Cruz-Merino, L.; Hajji, N.; Sánchez-Margalet, V.; Hontecillas-Prieto, L. Cancer Nano-Immunotherapy: The Novel and Promising Weapon to Fight Cancer. Int. J. Mol. Sci. 2024, 25, 1195. [Google Scholar] [CrossRef]
- Nasr-Eldahan, S.; Nabil-Adam, A.; Shreadah, M.A.; Maher, A.M.; Ali, T.E.S. A review article on nanotechnology in aquaculture sustainability as a novel tool in fish disease control. Aquacult. Int. 2021, 29, 1459–1480. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Mahanty, A.; Gupta, S.K.; Choudhury, A.R.; Daware, A.; Bhattacharjee, S. Nanotechnology: A next-generation tool for sustainable aquaculture. Aquaculture 2022, 546, 737330. [Google Scholar] [CrossRef]
- Madrid, R.R.M.; Mertins, O.; Tavares-Dias, M.; Flores-Gonzales, A.P.; Patta, A.C.M.F.; Ramirez, C.A.B.; Rigoni, V.L.S.; Mathews, P.D. High compliance and effective treatment of fish endoparasitic infections with oral drug delivery nanobioparticles: Safety of intestinal tissue and blood parameters. J. Fish Dis. 2021, 44, 1819–1829. [Google Scholar] [CrossRef]
- Mathews, P.D.; Patta, A.C.M.F.; Gonçalves, J.V.; Gama, G.D.S.; Garcia, I.T.S.; Mertins, O. Targeted Drug Delivery and Treatment of Endoparasites with Biocompatible Particles of pH-Responsive Structure. Biomacromolecules 2018, 19, 499–510. [Google Scholar] [CrossRef]
- Mathews, P.D.; Patta, A.C.M.F.; Madrid, R.R.M.; Ramirez, C.A.B.; Pimenta, B.V.; Mertins, O. Efficient Treatment of Fish Intestinal Parasites Applying a Membrane-Penetrating Oral Drug Delivery Nanoparticle. ACS Biomater. Sci. Eng. 2023, 9, 2911–2923. [Google Scholar] [CrossRef]
- Kabiri, M.; Unsworth, L.D. Application of Isothermal Titration Calorimetry for Characterizing Thermodynamic Parameters of Biomolecular Interactions: Peptide Self-Assembly and Protein Adsorption Case Studies. Biomacromolecules 2014, 15, 3463–3473. [Google Scholar] [CrossRef] [PubMed]
- Omanovic-Miklicanin, E.; Manfield, I.; Wilkins, T. Application of isothermal titration calorimetry in evaluation of protein–nanoparticle interactions. J. Therm. Anal. Calorim. 2017, 127, 605–613. [Google Scholar] [CrossRef]
- Archer, W.R.; Schulz, M.D. Isothermal titration calorimetry: Practical approaches and current applications in soft matter. Soft Matter 2020, 16, 8760–8774. [Google Scholar] [CrossRef]
- Abraham, T.; Lewis, R.N.A.H.; Hodges, R.S.; McElhaney, R.N. Isothermal Titration Calorimetry Studies of the Binding of a Rationally Designed Analogue of the Antimicrobial Peptide Gramicidin S to Phospholipid Bilayer Membranes. Biochemistry 2005, 44, 11279–11285. [Google Scholar] [CrossRef]
- Pelegrina, J.L.; Gennari, F.C.; Condo, A.M.; Guillermet, A.F. Predictive Gibbs-energy approach to crystalline/amorphous relative stability of nanoparticles: Size-effect calculations and experimental test. J. Alloys Comp. 2016, 689, 161–168. [Google Scholar] [CrossRef]
- Madrid, R.R.M.; Mathews, P.D.; Patta, A.C.M.F.; Gonzales-Flores, A.P.; Ramirez, C.A.B.; Rigoni, V.L.S.; Tavares-Dias, M.; Mertins, O. Safety of oral administration of high doses of ivermectin by means of biocompatible polyelectrolytes formulation. Heliyon 2021, 7, e05820. [Google Scholar] [CrossRef] [PubMed]
- Patta, A.C.M.F.; Mathews, P.D.; Madrid, R.R.M.; Rigoni, V.L.S.; Silva, E.R.; Mertins, O. Polyionic complexes of chitosan-N-arginine with alginate as pH responsive and mucoadhesive particles for oral drug delivery applications. Int. J. Biol. Macromol. 2020, 148, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.A.B.; Mathews, P.D.; Madrid, R.R.M.; Garcia, I.T.S.; Rigoni, V.L.S.; Mertins, O. Antibacterial polypeptide-bioparticle for oral administration: Powder formulation, palatability and in vivo toxicity approach. Biomat. Adv. 2023, 153, 213525. [Google Scholar] [CrossRef]
- Matthew, S.G.; Najahi-Missaoui, W. Lyophilization of Nanoparticles, Does It Really Work? Overview of the Current Status and Challenges. Int. J. Mol. Sci. 2023, 24, 14041. [Google Scholar] [CrossRef]
- Tella, A.C.; Olabemiwo, O.M.; Salawu, M.O.; Obiyenwa, G.K. Developing a spectrophotometric method for the estimation of albendazole in solid and suspension forms. Int. J. Phys. Sci. 2010, 5, 379–382. [Google Scholar]
- Philippova, O.E.; Volkov, E.V.; Sitnikova, N.L.; Khokhlov, A.R.; Desbrieres, J.; Rinaudo, M. Two Types of Hydrophobic Aggregates in Aqueous Solutions of Chitosan and Its Hydrophobic Derivative. Biomacromolecules 2001, 2, 483–490. [Google Scholar] [CrossRef]
- Madrid, R.R.M.; Mathews, P.D.; Pramanik, S.; Mangiarotti, A.; Fernandes, R.; Itri, R.; Dimova, R.; Mertins, O. Hybrid crystalline bioparticles with nanochannels encapsulating acemannan from Aloe vera: Structure and interaction with lipid membranes. J. Colloid Interface Sci. 2024, 673, 373–385. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Apeagyei, A.K.; Grenfell, J.R.A.; Airey, G.D. Application of Fickian and non-Fickian diffusion models to study moisture diffusion in asphalt mastics. Mater. Struct. 2015, 48, 1461–1474. [Google Scholar] [CrossRef]
- Delgado, R. Misuse of Beer-Lambert Law and other calibration curves. R. Soc. Open Sci. 2022, 9, 211103. [Google Scholar] [CrossRef] [PubMed]
- Cuadros, R.C.; Rivadeneyra, N.L.S.; Flores-Gonzales, A.; Mertins, O.; Malta, J.C.O.; Serrano-Martínez, M.E.; Mathews, P.D. Intestinal histological alterations in farmed red-bellied pacu Piaractus brachypomus (Characiformes: Serrasalmidae) heavily infected by roundworms. Aquacult. Int. 2021, 29, 989–998. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology Meets Ecology on Its Own Terms: Margolis et al. Revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
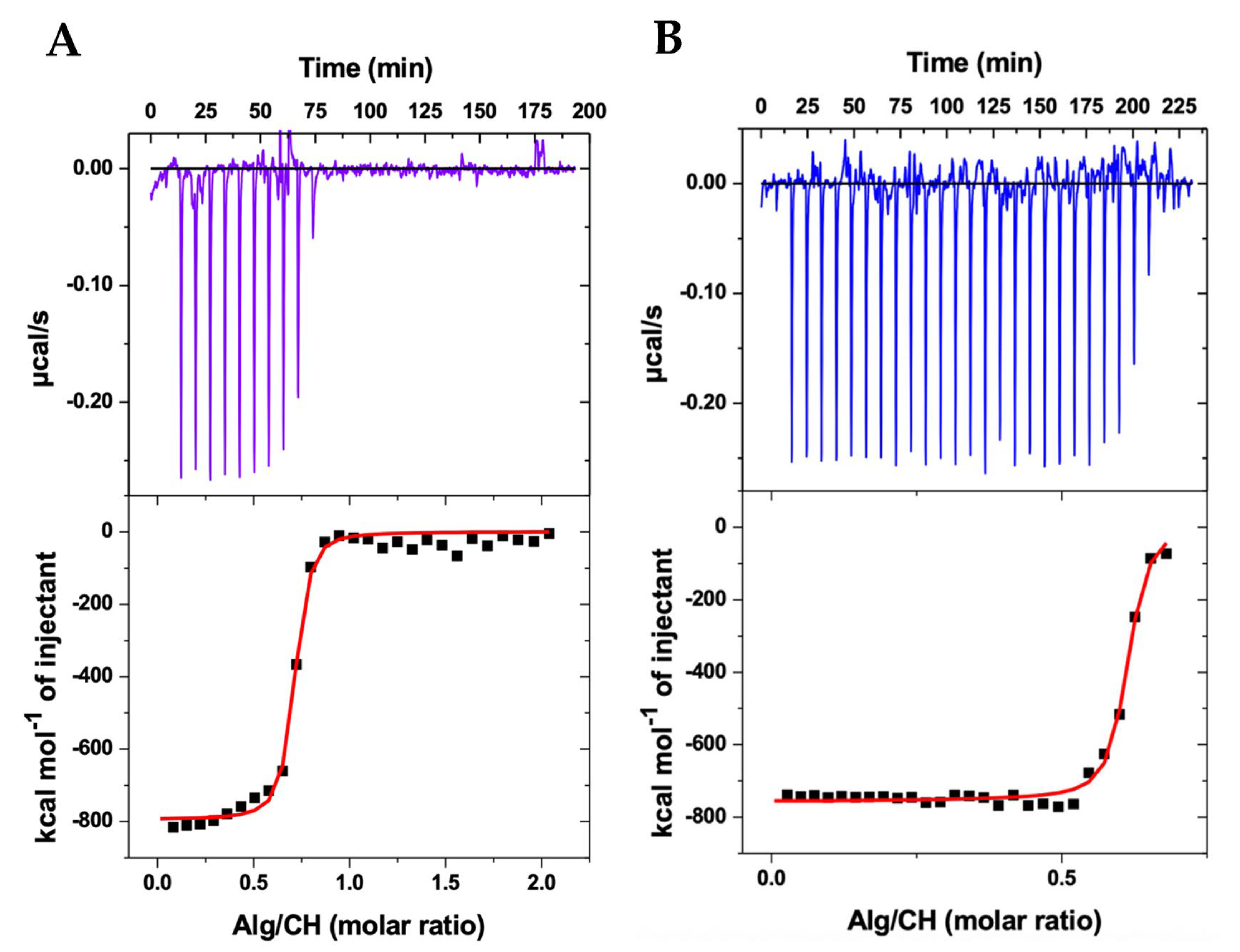
| Sample | N | K (109 M−1) | ΔH (kcal/mol) | ΔG (kcal/mol) | TΔS (kcal/mol) |
|---|---|---|---|---|---|
| CHAlg Alb | 0.682 ± 0.005 | 5.02 ± 1.31 | −794 ± 11 | –15.6 | 778 |
| CHAlg | 0.601 ± 0.002 | 7.60 ± 1.22 | −756 ± 43 | –15.9 | 740 |
| Nanoparticle | Hydrodynamic Diameter (nm) | PDI | Zeta Potential (mV) | Conductivity (mS/cm) |
|---|---|---|---|---|
| CHAlg | 382 ± 37 | 0.28 | 2.0 ± 2.7 | 0.62 |
| CHAlg-Alb | 475 ± 51 | 0.33 | 9.5 ± 3.2 | 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Rengifo, A.V.C.; Rivadeneyra Sánchez, N.L.; Teixeira, C.B.; Madrid, R.R.M.; Mertins, O.; Mathews, P.D. Coacervated and Freeze-Dried Polysaccharides-Nanoparticle with Efficient Encapsulation of Albendazole for High-Performance Treatment of Monogenean Parasite Infestation in Tilapia Fish. Int. J. Mol. Sci. 2026, 27, 1001. https://doi.org/10.3390/ijms27021001
Rengifo AVC, Rivadeneyra Sánchez NL, Teixeira CB, Madrid RRM, Mertins O, Mathews PD. Coacervated and Freeze-Dried Polysaccharides-Nanoparticle with Efficient Encapsulation of Albendazole for High-Performance Treatment of Monogenean Parasite Infestation in Tilapia Fish. International Journal of Molecular Sciences. 2026; 27(2):1001. https://doi.org/10.3390/ijms27021001
Chicago/Turabian StyleRengifo, Andrés Vicent Cubas, Norma Lorena Rivadeneyra Sánchez, Chloé Barbosa Teixeira, Rafael R. M. Madrid, Omar Mertins, and Patrick D. Mathews. 2026. "Coacervated and Freeze-Dried Polysaccharides-Nanoparticle with Efficient Encapsulation of Albendazole for High-Performance Treatment of Monogenean Parasite Infestation in Tilapia Fish" International Journal of Molecular Sciences 27, no. 2: 1001. https://doi.org/10.3390/ijms27021001
APA StyleRengifo, A. V. C., Rivadeneyra Sánchez, N. L., Teixeira, C. B., Madrid, R. R. M., Mertins, O., & Mathews, P. D. (2026). Coacervated and Freeze-Dried Polysaccharides-Nanoparticle with Efficient Encapsulation of Albendazole for High-Performance Treatment of Monogenean Parasite Infestation in Tilapia Fish. International Journal of Molecular Sciences, 27(2), 1001. https://doi.org/10.3390/ijms27021001








