The Double-Edged Nature of Methyl Donors in Cancer Development from Prevention to Progression
Abstract
1. Introduction
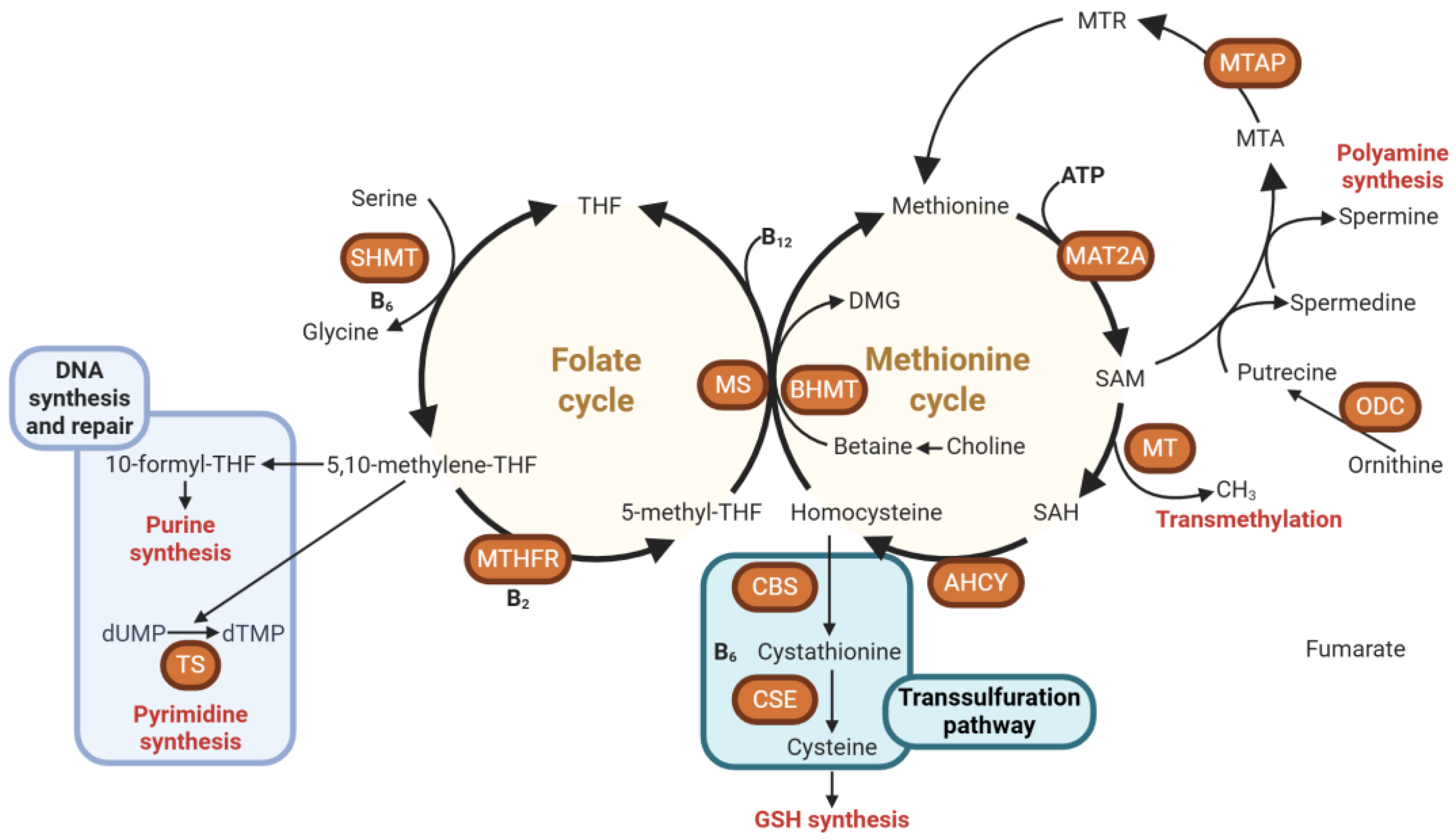
2. Overview of Methyl Donors and One-Carbon Metabolism
2.1. Key Nutrients and Pathways
2.2. Regulation of DNA and Histone Methylation
2.3. Interaction with Other Nutrients and Cofactors
3. The Dual Role of Methyl Donors in Cancer: From Prevention to Progression
3.1. Methyl Donors in Cancer Prevention: Safeguarding Genomic Stability Through Epigenetic Regulation
3.2. Methyl Donors and Cancer Progression: How Over-Supply Can Drive Tumorigenesis
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niculescu, M.D.; Zeisel, S.H. Diet, methyl donors and DNA methylation: Interactions between dietary folate, methionine and choline. J. Nutr. 2002, 132, 2333s–2335s. [Google Scholar] [CrossRef] [PubMed]
- Bekdash, R.A. Methyl Donors, Epigenetic Alterations, and Brain Health: Understanding the Connection. Int. J. Mol. Sci. 2023, 24, 2346. [Google Scholar] [CrossRef]
- Zhou, D.; Robertson, K.D. Chapter 24—Role of DNA Methylation in Genome Stability. In Genome Stability; Kovalchuk, I., Kovalchuk, O., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 409–424. [Google Scholar]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Geissler, F.; Nesic, K.; Kondrashova, O.; Dobrovic, A.; Swisher, E.M.; Scott, C.L.; Wakefield, M.J. The role of aberrant DNA methylation in cancer initiation and clinical impacts. Ther. Adv. Med. Oncol. 2024, 16, 17588359231220511. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Ali, M.M. Methyl Donor Micronutrients that Modify DNA Methylation and Cancer Outcome. Nutrients 2019, 11, 608. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Su, M.; Huang, G.; Luo, P.; Zhang, T.; Fu, L.; Wei, J.; Wang, S.; Sun, G. MTHFR C677T genetic polymorphism in combination with serum vitamin B2, B12 and aberrant DNA methylation of P16 and P53 genes in esophageal squamous cell carcinoma and esophageal precancerous lesions: A case-control study. Cancer Cell Int. 2019, 19, 288. [Google Scholar] [CrossRef]
- Korsmo, H.W.; Jiang, X. One carbon metabolism and early development: A diet-dependent destiny. Trends Endocrinol. Metab. 2021, 32, 579–593. [Google Scholar] [CrossRef]
- Bailey, L.B.; Gregory, J.F., 3rd. Polymorphisms of methylenetetrahydrofolate reductase and other enzymes: Metabolic significance, risks and impact on folate requirement. J. Nutr. 1999, 129, 919–922. [Google Scholar] [CrossRef]
- Xing, Z.; Tu, B.P. Mechanisms and rationales of SAM homeostasis. Trends Biochem. Sci. 2025, 50, 242–254. [Google Scholar] [CrossRef]
- Mato, J.M.; Martinez-Chantar, M.L.; Lu, S.C. S-adenosylmethionine metabolism and liver disease. Ann. Hepatol. 2013, 12, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, A.; Beccarini Crescenzi, B.; Bolognesi, S.; Goracci, A.; Koukouna, D.; Rossi, R.; Fagiolini, A. S-Adenosylmethionine (SAMe) in major depressive disorder (MDD): A clinician-oriented systematic review. Ann. Gen. Psychiatry 2020, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Caudill, M.A.; Wang, J.C.; Melnyk, S.; Pogribny, I.P.; Jernigan, S.; Collins, M.D.; Santos-Guzman, J.; Swendseid, M.E.; Cogger, E.A.; James, S.J. Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine beta-synthase heterozygous mice. J. Nutr. 2001, 131, 2811–2818. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2011, 34, 3–15. [Google Scholar] [CrossRef]
- Zeisel, S. Choline, Other Methyl-Donors and Epigenetics. Nutrients 2017, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.W.; Kinosian, B.P.; Stoner, N.E.; Lentine, D.C.; Buzby, G.P. Choline and vitamin B12 deficiencies are interrelated in folate-replete long-term total parenteral nutrition patients. JPEN J. Parenter. Enter. Nutr. 2002, 26, 57–62. [Google Scholar] [CrossRef]
- Carballal, S.; Banerjee, R. Chapter 19—Overview of cysteine metabolism. In Redox Chemistry and Biology of Thiols; Alvarez, B., Comini, M.A., Salinas, G., Trujillo, M., Eds.; Academic Press: Boston, MA, USA, 2022; pp. 423–450. [Google Scholar]
- Petrova, B.; Maynard, A.G.; Wang, P.; Kanarek, N. Regulatory mechanisms of one-carbon metabolism enzymes. J. Biol. Chem. 2023, 299, 105457. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial one-carbon metabolism maintains redox balance during hypoxia. Cancer Discov. 2014, 4, 1371–1373. [Google Scholar] [CrossRef]
- Jędrzejewski, M.; Szeleszczuk, Ł.; Pisklak, D.M. Mechanistic Insights into SAM-Dependent Methyltransferases: A Review of Computational Approaches. Int. J. Mol. Sci. 2025, 26, 9204. [Google Scholar] [CrossRef]
- Li, J.; Sun, C.; Cai, W.; Li, J.; Rosen, B.P.; Chen, J. Insights into S-adenosyl-l-methionine (SAM)-dependent methyltransferase related diseases and genetic polymorphisms. Mutat. Res./Rev. Mutat. Res. 2021, 788, 108396. [Google Scholar] [CrossRef]
- Struck, A.W.; Thompson, M.L.; Wong, L.S.; Micklefield, J. S-adenosyl-methionine-dependent methyltransferases: Highly versatile enzymes in biocatalysis, biosynthesis and other biotechnological applications. ChemBioChem 2012, 13, 2642–2655. [Google Scholar] [CrossRef]
- Greer, E.L.; Shi, Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012, 13, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Pekowska, A.; Benoukraf, T.; Zacarias-Cabeza, J.; Belhocine, M.; Koch, F.; Holota, H.; Imbert, J.; Andrau, J.C.; Ferrier, P.; Spicuglia, S. H3K4 tri-methylation provides an epigenetic signature of active enhancers. EMBO J. 2011, 30, 4198–4210. [Google Scholar] [CrossRef]
- Asai, A.; Konno, M.; Koseki, J.; Taniguchi, M.; Vecchione, A.; Ishii, H. One-carbon metabolism for cancer diagnostic and therapeutic approaches. Cancer Lett. 2020, 470, 141–148. [Google Scholar] [CrossRef]
- Newman, A.C.; Maddocks, O.D.K. One-carbon metabolism in cancer. Br. J. Cancer 2017, 116, 1499–1504. [Google Scholar] [CrossRef]
- Aragão, M.Â.; Pires, L.; Santos-Buelga, C.; Barros, L.; Calhelha, R.C. Revitalising Riboflavin: Unveiling Its Timeless Significance in Human Physiology and Health. Foods 2024, 13, 2255. [Google Scholar] [CrossRef]
- Gregory, J.F.; DeRatt, B.N.; Rios-Avila, L.; Ralat, M.; Stacpoole, P.W. Vitamin B6 nutritional status and cellular availability of pyridoxal 5′-phosphate govern the function of the transsulfuration pathway’s canonical reactions and hydrogen sulfide production via side reactions. Biochimie 2016, 126, 21–26. [Google Scholar] [CrossRef]
- Brito, S.; Lee, M.G.; Bin, B.H.; Lee, J.S. Zinc and Its Transporters in Epigenetics. Mol. Cells 2020, 43, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Nejdl, L.; Ruttkay-Nedecky, B.; Kudr, J.; Krizkova, S.; Smerkova, K.; Dostalova, S.; Vaculovicova, M.; Kopel, P.; Zehnalek, J.; Trnkova, L.; et al. DNA interaction with zinc(II) ions. Int. J. Biol. Macromol. 2014, 64, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Qin, J.; Liu, M.; Lu, M.; Dong, H.; Liu, D.; Li, Y.; Lu, K.; Wei, L.; Ma, L. Biosynthesis and bioassays of multifunctional S-adenosylmethionine: A comprehensive review. Chem. Eng. J. 2025, 519, 164933. [Google Scholar] [CrossRef]
- Obeid, R. High Plasma Vitamin B12 and Cancer in Human Studies: A Scoping Review to Judge Causality and Alternative Explanations. Nutrients 2022, 14, 4476. [Google Scholar] [CrossRef] [PubMed]
- Mayne, S.T.; Risch, H.A.; Dubrow, R.; Chow, W.H.; Gammon, M.D.; Vaughan, T.L.; Farrow, D.C.; Schoenberg, J.B.; Stanford, J.L.; Ahsan, H.; et al. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol. Biomark. Prev. 2001, 10, 1055–1062. [Google Scholar]
- Chang, S.C.; Goldstein, B.Y.; Mu, L.; Cai, L.; You, N.C.; He, N.; Ding, B.G.; Zhao, J.K.; Yu, S.Z.; Heber, D.; et al. Plasma folate, vitamin B12, and homocysteine and cancers of the esophagus, stomach, and liver in a Chinese population. Nutr. Cancer 2015, 67, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Li, Q.; Xin, Y.; Fang, X.; Tian, Y.; Ma, J.; Wang, J.; Wang, Q.; Zhang, R.; Wang, J.; et al. Intake of Dietary One-Carbon Metabolism-Related B Vitamins and the Risk of Esophageal Cancer: A Dose-Response Meta-Analysis. Nutrients 2018, 10, 835. [Google Scholar] [CrossRef]
- Miranti, E.H.; Stolzenberg-Solomon, R.; Weinstein, S.J.; Selhub, J.; Mannisto, S.; Taylor, P.R.; Freedman, N.D.; Albanes, D.; Abnet, C.C.; Murphy, G. Low vitamin B12 increases risk of gastric cancer: A prospective study of one-carbon metabolism nutrients and risk of upper gastrointestinal tract cancer. Int. J. Cancer 2017, 141, 1120–1129. [Google Scholar] [CrossRef]
- Xiao, Q.; Freedman, N.D.; Ren, J.; Hollenbeck, A.R.; Abnet, C.C.; Park, Y. Intakes of folate, methionine, vitamin B6, and vitamin B12 with risk of esophageal and gastric cancer in a large cohort study. Br. J. Cancer 2014, 110, 1328–1333. [Google Scholar] [CrossRef]
- Wainfan, E.; Dizik, M.; Stender, M.; Christman, J.K. Rapid Appearance of Hypomethylated DNA in Livers of Rats Fed Cancerpromoting, Methyl-deficient Diets1. Cancer Res. 1989, 49, 4094–4097. [Google Scholar]
- Ulrich, C.M.; Potter, J.D. Folate supplementation: Too much of a good thing? Cancer Epidemiol. Biomark. Prev. 2006, 15, 189–193. [Google Scholar] [CrossRef]
- Deng, L.; Huang-fu, Y.-C.; Ma, Y.-H. Dietary nutrients involved in one-carbon metabolism and colorectal cancer risk. LabMed Discov. 2024, 1, 100022. [Google Scholar] [CrossRef]
- Cartron, P.F.; Hervouet, E.; Debien, E.; Olivier, C.; Pouliquen, D.; Menanteau, J.; Loussouarn, D.; Martin, S.A.; Campone, M.; Vallette, F.M. Folate supplementation limits the tumourigenesis in rodent models of gliomagenesis. Eur. J. Cancer 2012, 48, 2431–2441. [Google Scholar] [CrossRef]
- Eden, A.; Gaudet, F.; Waghmare, A.; Jaenisch, R. Chromosomal instability and tumors promoted by DNA hypomethylation. Science 2003, 300, 455. [Google Scholar] [CrossRef] [PubMed]
- Hervouet, E.; Lalier, L.; Debien, E.; Cheray, M.; Geairon, A.; Rogniaux, H.; Loussouarn, D.; Martin, S.A.; Vallette, F.M.; Cartron, P.F. Disruption of Dnmt1/PCNA/UHRF1 interactions promotes tumorigenesis from human and mice glial cells. PLoS ONE 2010, 5, e11333. [Google Scholar] [CrossRef]
- Keyes, M.K.; Jang, H.; Mason, J.B.; Liu, Z.; Crott, J.W.; Smith, D.E.; Friso, S.; Choi, S.-W. Older Age and Dietary Folate Are Determinants of Genomic and p16-Specific DNA Methylation in Mouse Colon12. J. Nutr. 2007, 137, 1713–1717. [Google Scholar] [CrossRef]
- Wang, S.; Pan, D.; Su, M.; Huang, G.; Sun, G. Moderately high folate level may offset the effects of aberrant DNA methylation of P16 and P53 genes in esophageal squamous cell carcinoma and precancerous lesions. Genes Nutr. 2020, 15, 18. [Google Scholar] [CrossRef]
- Pan, D.; Su, M.; Xu, D.; Wang, Y.; Gao, H.; Smith, J.D.; Sun, J.; Wang, X.; Yan, Q.; Song, G.; et al. Exploring the Interplay Between Vitamin B(12)-related Biomarkers, DNA Methylation, and Gene-Nutrition Interaction in Esophageal Precancerous Lesions. Arch. Med. Res. 2023, 54, 102889. [Google Scholar] [CrossRef]
- Pan, D.; Wang, S.; Su, M.; Sun, G.; Zhu, X.; Ghahvechi Chaeipeima, M.; Guo, Z.; Wang, N.; Zhang, Z.; Cui, M. Vitamin B12 may play a preventive role in esophageal precancerous lesions: A case–control study based on markers in blood and 3-day duplicate diet samples. Eur. J. Nutr. 2021, 60, 3375–3386. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.M.; Kamynina, E.; Field, M.S.; Stover, P.J. Folate rescues vitamin B(12) depletion-induced inhibition of nuclear thymidylate biosynthesis and genome instability. Proc. Natl. Acad. Sci. USA 2017, 114, E4095–E4102. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.M.; Weir, D.G. The methyl folate trap. A physiological response in man to prevent methyl group deficiency in kwashiorkor (methionine deficiency) and an explanation for folic-acid induced exacerbation of subacute combined degeneration in pernicious anaemia. Lancet 1981, 2, 337–340. [Google Scholar] [CrossRef]
- Kutzbach, C.; Stokstad, E.L. Mammalian methylenetetrahydrofolate reductase. Partial purification, properties, and inhibition by S-adenosylmethionine. Biochim. Biophys. Acta 1971, 250, 459–477. [Google Scholar] [CrossRef]
- Choi, S.W.; Friso, S.; Ghandour, H.; Bagley, P.J.; Selhub, J.; Mason, J.B. Vitamin B-12 deficiency induces anomalies of base substitution and methylation in the DNA of rat colonic epithelium. J. Nutr. 2004, 134, 750–755. [Google Scholar] [CrossRef]
- Kulkarni, A.; Dangat, K.; Kale, A.; Sable, P.; Chavan-Gautam, P.; Joshi, S. Effects of altered maternal folic acid, vitamin B12 and docosahexaenoic acid on placental global DNA methylation patterns in Wistar rats. PLoS ONE 2011, 6, e17706. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, K.D.; Allegrucci, C.; Singh, R.; Gardner, D.S.; Sebastian, S.; Bispham, J.; Thurston, A.; Huntley, J.F.; Rees, W.D.; Maloney, C.A.; et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc. Natl. Acad. Sci. USA 2007, 104, 19351–19356. [Google Scholar] [CrossRef]
- Mocellin, S.; Briarava, M.; Pilati, P. Vitamin B6 and Cancer Risk: A Field Synopsis and Meta-Analysis. J. Natl. Cancer Inst. 2017, 109, djw230. [Google Scholar] [CrossRef] [PubMed]
- Huq, M.D.; Tsai, N.P.; Lin, Y.P.; Higgins, L.; Wei, L.N. Vitamin B6 conjugation to nuclear corepressor RIP140 and its role in gene regulation. Nat. Chem. Biol. 2007, 3, 161–165. [Google Scholar] [CrossRef]
- Frost, Z.; Bakhit, S.; Amaefuna, C.N.; Powers, R.V.; Ramana, K.V. Recent Advances on the Role of B Vitamins in Cancer Prevention and Progression. Int. J. Mol. Sci. 2025, 26, 1967. [Google Scholar] [CrossRef]
- Pilesi, E.; Tesoriere, G.; Ferriero, A.; Mascolo, E.; Liguori, F.; Argiro, L.; Angioli, C.; Tramonti, A.; Contestabile, R.; Volonte, C.; et al. Vitamin B6 deficiency cooperates with oncogenic Ras to induce malignant tumors in Drosophila. Cell Death Dis. 2024, 15, 388. [Google Scholar] [CrossRef]
- Tesoriere, G.; Pilesi, E.; De Rosa, M.; Giampaoli, O.; Patriarca, A.; Spagnoli, M.; Chiocciolini, F.; Tramonti, A.; Contestabile, R.; Sciubba, F.; et al. Vitamin B6 deficiency produces metabolic alterations in Drosophila. Metabolomics 2025, 21, 42. [Google Scholar] [CrossRef]
- Marzio, A.; Merigliano, C.; Gatti, M.; Verni, F. Sugar and chromosome stability: Clastogenic effects of sugars in vitamin B6-deficient cells. PLoS Genet. 2014, 10, e1004199. [Google Scholar] [CrossRef]
- Spinneker, A.; Sola, R.; Lemmen, V.; Castillo, M.J.; Pietrzik, K.; Gonzalez-Gross, M. Vitamin B6 status, deficiency and its consequences—An overview. Nutr. Hosp. 2007, 22, 7–24. [Google Scholar] [PubMed]
- Yasuda, H.; Tsutsui, M.; Ando, J.; Inano, T.; Noguchi, M.; Yahata, Y.; Tanaka, M.; Tsukune, Y.; Masuda, A.; Shirane, S.; et al. Vitamin B6 deficiency is prevalent in primary and secondary myelofibrosis patients. Int. J. Hematol. 2019, 110, 543–549. [Google Scholar] [CrossRef]
- Sun, S.; Li, X.; Ren, A.; Du, M.; Du, H.; Shu, Y.; Zhu, L.; Wang, W. Choline and betaine consumption lowers cancer risk: A meta-analysis of epidemiologic studies. Sci. Rep. 2016, 6, 35547. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.; Cho, E.; Lee, J.E. Association of choline and betaine levels with cancer incidence and survival: A meta-analysis. Clin. Nutr. 2019, 38, 100–109. [Google Scholar] [CrossRef]
- Gomez, M.F.; Hogue, S.R.; Salemi, J.L.; Gray, H.L.; Kanetsky, P.A.; Liao, L.M.; Alman, A.C.; Sinha, R.; Byrd, D.A. Associations of Dietary Choline and Betaine with Colorectal Cancer Incidence in the NIH-AARP Diet and Health Cohort. Curr. Dev. Nutr. 2025, 9, 106514. [Google Scholar] [CrossRef]
- Lee, J.E.; Jacques, P.F.; Dougherty, L.; Selhub, J.; Giovannucci, E.; Zeisel, S.H.; Cho, E. Are dietary choline and betaine intakes determinants of total homocysteine concentration? Am. J. Clin. Nutr. 2010, 91, 1303–1310. [Google Scholar] [CrossRef]
- Nitter, M.; Norgard, B.; de Vogel, S.; Eussen, S.J.; Meyer, K.; Ulvik, A.; Ueland, P.M.; Nygard, O.; Vollset, S.E.; Bjorge, T.; et al. Plasma methionine, choline, betaine, and dimethylglycine in relation to colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). Ann. Oncol. 2014, 25, 1609–1615. [Google Scholar] [CrossRef]
- Pogribny, I.P.; James, S.J.; Beland, F.A. Molecular alterations in hepatocarcinogenesis induced by dietary methyl deficiency. Mol. Nutr. Food Res. 2012, 56, 116–125. [Google Scholar] [CrossRef]
- Shivapurkar, N.; Poirier, L.A. Tissue levels of S-adenosylmethionine and S-adenosylhomocysteine in rats fed methyl-deficient, amino acid-defined diets for one to five weeks. Carcinogenesis 1983, 4, 1051–1057. [Google Scholar] [CrossRef]
- Tehlivets, O.; Malanovic, N.; Visram, M.; Pavkov-Keller, T.; Keller, W. S-adenosyl-L-homocysteine hydrolase and methylation disorders: Yeast as a model system. Biochim. Biophys. Acta 2013, 1832, 204–215. [Google Scholar] [CrossRef]
- Lin, N.; Qin, S.; Luo, S.; Cui, S.; Huang, G.; Zhang, X. Homocysteine induces cytotoxicity and proliferation inhibition in neural stem cells via DNA methylation in vitro. FEBS J. 2014, 281, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Wainfan, E.; Poirier, L.A. Methyl groups in carcinogenesis: Effects on DNA methylation and gene expression. Cancer Res. 1992, 52, 2071s–2077s. [Google Scholar] [PubMed]
- Tsujiuchi, T.; Tsutsumi, M.; Sasaki, Y.; Takahama, M.; Konishi, Y. Hypomethylation of CpG sites and c-myc gene overexpression in hepatocellular carcinomas, but not hyperplastic nodules, induced by a choline-deficient L-amino acid-defined diet in rats. Jpn. J. Cancer Res. 1999, 90, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Tryndyak, V.P.; Han, T.; Muskhelishvili, L.; Fuscoe, J.C.; Ross, S.A.; Beland, F.A.; Pogribny, I.P. Coupling global methylation and gene expression profiles reveal key pathophysiological events in liver injury induced by a methyl-deficient diet. Mol. Nutr. Food Res. 2011, 55, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Lupu, D.S.; Orozco, L.D.; Wang, Y.; Cullen, J.M.; Pellegrini, M.; Zeisel, S.H. Altered methylation of specific DNA loci in the liver of Bhmt-null mice results in repression of Iqgap2 and F2rl2 and is associated with development of preneoplastic foci. FASEB J. 2017, 31, 2090–2103. [Google Scholar] [CrossRef]
- Zeisel, S.H.; da Costa, K.A.; Albright, C.D.; Shin, O.H. Choline and hepatocarcinogenesis in the rat. Diet Cancer 1995, 375, 65–74. [Google Scholar] [CrossRef]
- da Costa, K.A.; Cochary, E.F.; Blusztajn, J.K.; Garner, S.C.; Zeisel, S.H. Accumulation of 1,2-sn-diradylglycerol with increased membrane-associated protein kinase C may be the mechanism for spontaneous hepatocarcinogenesis in choline-deficient rats. J. Biol. Chem. 1993, 268, 2100–2105. [Google Scholar] [CrossRef] [PubMed]
- da Costa, K.A.; Garner, S.C.; Chang, J.; Zeisel, S.H. Effects of prolonged (1 year) choline deficiency and subsequent re-feeding of choline on 1,2-sn-diradylglycerol, fatty acids and protein kinase C in rat liver. Carcinogenesis 1995, 16, 327–334. [Google Scholar] [CrossRef]
- Du, Y.P.; Peng, J.S.; Sun, A.; Tang, Z.H.; Ling, W.H.; Zhu, H.L. Assessment of the effect of betaine on p16 and c-myc DNA methylation and mRNA expression in a chemical induced rat liver cancer model. BMC Cancer 2009, 9, 261. [Google Scholar] [CrossRef]
- Finkelstein, J.D.; Martin, J.J. Methionine metabolism in mammals. Adaptation to methionine excess. J. Biol. Chem. 1986, 261, 1582–1587. [Google Scholar] [CrossRef]
- Regina, M.; Korhonen, V.P.; Smith, T.K.; Alakuijala, L.; Eloranta, T.O. Methionine toxicity in the rat in relation to hepatic accumulation of S-adenosylmethionine: Prevention by dietary stimulation of the hepatic transsulfuration pathway. Arch. Biochem. Biophys. 1993, 300, 598–607. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Wan, X.Y.; Cao, J.W. Dietary methionine intake and risk of incident colorectal cancer: A meta-analysis of 8 prospective studies involving 431,029 participants. PLoS ONE 2013, 8, e83588. [Google Scholar] [CrossRef]
- Vidal, A.C.; Grant, D.J.; Williams, C.D.; Masko, E.; Allott, E.H.; Shuler, K.; McPhail, M.; Gaines, A.; Calloway, E.; Gerber, L.; et al. Associations between Intake of Folate, Methionine, and Vitamins B-12, B-6 and Prostate Cancer Risk in American Veterans. J. Cancer Epidemiol. 2012, 2012, 957467. [Google Scholar] [CrossRef]
- Feigelson, H.S.; Jonas, C.R.; Robertson, A.S.; McCullough, M.L.; Thun, M.J.; Calle, E.E. Alcohol, folate, methionine, and risk of incident breast cancer in the American Cancer Society Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol. Biomark. Prev. 2003, 12, 161–164. [Google Scholar]
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and epigenetics: An interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, W.; Peng, Y.; Zhao, C.; Ren, J.; Qu, X. Precise Targeting One-Carbon Metabolism for Potent Cancer Therapy and Metastasis Suppression. Small 2025, 21, e04631. [Google Scholar] [CrossRef]
- Kory, N.; Wyant, G.A.; Prakash, G.; Uit de Bos, J.; Bottanelli, F.; Pacold, M.E.; Chan, S.H.; Lewis, C.A.; Wang, T.; Keys, H.R.; et al. SFXN1 is a mitochondrial serine transporter required for one-carbon metabolism. Science 2018, 362, eaat9528. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Vousden, K.H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 2016, 16, 650–662. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Gigic, B.; van Roekel, E.; Holowatyj, A.N.; Brezina, S.; Geijsen, A.; Ulvik, A.; Ose, J.; Koole, J.L.; Damerell, V.; Kiblawi, R.; et al. Cohort profile: Biomarkers related to folate-dependent one-carbon metabolism in colorectal cancer recurrence and survival—The FOCUS Consortium. BMJ Open 2022, 12, e062930. [Google Scholar] [CrossRef]
- Weißenborn, A.; Ehlers, A.; Hirsch-Ernst, K.I.; Lampen, A.; Niemann, B. A two-faced vitamin: Folic acid—Prevention or promotion of colon cancer? Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2017, 60, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Medline, A.; Mason, J.B.; Gallinger, S.; Kim, Y.I. Effects of dietary folate on intestinal tumorigenesis in the apcMin mouse. Cancer Res. 2000, 60, 5434–5440. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Sohn, K.J.; Medline, A.; Ash, C.; Gallinger, S.; Kim, Y.I. Chemopreventive effects of dietary folate on intestinal polyps in Apc+/−Msh2−/− mice. Cancer Res. 2000, 60, 3191–3199. [Google Scholar]
- Kamen, B. Folate and antifolate pharmacology. Semin. Oncol. 1997, 24, S18–30–s18–39. [Google Scholar]
- Diaz, G.S.; LeBlanc, D.P.; Gagné, R.; Behan, N.A.; Wong, A.; Marchetti, F.; MacFarlane, A.J. Folate Intake Alters Mutation Frequency and Profiles in a Tissue- and Dose-Specific Manner in MutaMouse Male Mice. J. Nutr. 2021, 151, 800–809. [Google Scholar] [CrossRef]
- Rosen, F.; Nichol, C.A. Inhibition of the growth of an ame-thopterin-refractory tumor by dietary restriction of folic acid. Cancer Res. 1962, 22, 495–500. [Google Scholar] [PubMed]
- Engelbreth-Holm, J.; Rask-Nielsen, R.; Hoff-JØRgensen, E.; Kalckar, H. The growth of Rous sarcoma in folic acid deficient chicks. Acta Pathol. Microbiol. Scand. 1951, 29, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Bills, N.D.; Hinrichs, S.H.; Morgan, R.; Clifford, A.J. Delayed tumor onset in transgenic mice fed a low-folate diet. J. Natl. Cancer Inst. 1992, 84, 332–337. [Google Scholar] [CrossRef]
- Farber, S. Some observations on the effect of folic acid antagonists on acute leukemia and other forms of incurable cancer. Blood 1949, 4, 160–167. [Google Scholar] [CrossRef]
- Ebbing, M.; Bønaa, K.H.; Nygård, O.; Arnesen, E.; Ueland, P.M.; Nordrehaug, J.E.; Rasmussen, K.; Njølstad, I.; Refsum, H.; Nilsen, D.W.; et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA 2009, 302, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Oliai Araghi, S.; Kiefte-de Jong, J.C.; van Dijk, S.C.; Swart, K.M.A.; van Laarhoven, H.W.; van Schoor, N.M.; de Groot, L.; Lemmens, V.; Stricker, B.H.; Uitterlinden, A.G.; et al. Folic Acid and Vitamin B12 Supplementation and the Risk of Cancer: Long-term Follow-up of the B Vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol. Biomark. Prev. 2019, 28, 275–282. [Google Scholar] [CrossRef]
- Kok, D.E.; Dhonukshe-Rutten, R.A.; Lute, C.; Heil, S.G.; Uitterlinden, A.G.; van der Velde, N.; van Meurs, J.B.; van Schoor, N.M.; Hooiveld, G.J.; de Groot, L.C.; et al. The effects of long-term daily folic acid and vitamin B12 supplementation on genome-wide DNA methylation in elderly subjects. Clin. Epigenetics 2015, 7, 121. [Google Scholar] [CrossRef]
- Jiang, S. Vitamin B6 Fuels Acute Myeloid Leukemia Growth. Trends Cancer 2020, 6, 536–537. [Google Scholar] [CrossRef]
- Chen, C.C.; Li, B.; Millman, S.E.; Chen, C.; Li, X.; Morris, J.P.; Mayle, A.; Ho, Y.J.; Loizou, E.; Liu, H.; et al. Vitamin B6 Addiction in Acute Myeloid Leukemia. Cancer Cell 2020, 37, 71–84.e7. [Google Scholar] [CrossRef]
- He, C.; Wang, D.; Shukla, S.K.; Hu, T.; Thakur, R.; Fu, X.; King, R.J.; Kollala, S.S.; Attri, K.S.; Murthy, D.; et al. Vitamin B6 Competition in the Tumor Microenvironment Hampers Antitumor Functions of NK Cells. Cancer Discov. 2024, 14, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Brasky, T.M.; White, E.; Chen, C.L. Long-Term, Supplemental, One-Carbon Metabolism-Related Vitamin B Use in Relation to Lung Cancer Risk in the Vitamins and Lifestyle (VITAL) Cohort. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3440–3448. [Google Scholar] [CrossRef]
- Corbin, J.M.; Ruiz-Echevarria, M.J. One-Carbon Metabolism in Prostate Cancer: The Role of Androgen Signaling. Int. J. Mol. Sci. 2016, 17, 1208. [Google Scholar] [CrossRef] [PubMed]
- Hanley, M.P.; Kadaveru, K.; Perret, C.; Giardina, C.; Rosenberg, D.W. Dietary Methyl Donor Depletion Suppresses Intestinal Adenoma Development. Cancer Prev. Res. 2016, 9, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Kadaveru, K.; Protiva, P.; Greenspan, E.J.; Kim, Y.I.; Rosenberg, D.W. Dietary methyl donor depletion protects against intestinal tumorigenesis in Apc(Min/+) mice. Cancer Prev. Res. 2012, 5, 911–920. [Google Scholar] [CrossRef]
- Hanley, M.P.; Aladelokun, O.; Kadaveru, K.; Rosenberg, D.W. Methyl Donor Deficiency Blocks Colorectal Cancer Development by Affecting Key Metabolic Pathways. Cancer Prev. Res. 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Hoffman, R.M. Is the Hoffman Effect for Methionine Overuse Analogous to the Warburg Effect for Glucose Overuse in Cancer? In Methionine Dependence of Cancer and Aging: Methods and Protocols; Hoffman, R.M., Ed.; Springer: New York, NY, USA, 2019; pp. 273–278. [Google Scholar]
- Bandaru, N.; Noor, S.M.; Kammili, M.L.; Bonthu, M.G.; Gayatri, A.P.; Kumar, P.K. Methionine restriction for cancer therapy: From preclinical studies to clinical trials. Cancer Pathog. Ther. 2025, in press. [Google Scholar] [CrossRef]
- Ma, C.; Xu, A.; Zuo, L.; Li, Q.; Fan, F.; Hu, Y.; Sun, C. Methionine Dependency and Restriction in Cancer: Exploring the Pathogenic Function and Therapeutic Potential. Pharmaceuticals 2025, 18, 640. [Google Scholar] [CrossRef]
- Garg, S.; Morehead, L.C.; Bird, J.T.; Graw, S.; Gies, A.; Storey, A.J.; Tackett, A.J.; Edmondson, R.D.; Mackintosh, S.G.; Byrum, S.D.; et al. Characterization of methionine dependence in melanoma cells. Mol. Omics 2023, 20, 37–47. [Google Scholar] [CrossRef]
- Wanders, D.; Hobson, K.; Ji, X. Methionine Restriction and Cancer Biology. Nutrients 2020, 12, 684. [Google Scholar] [CrossRef]
- Bin, P.; Wang, C.; Zhang, H.; Yan, Y.; Ren, W. Targeting methionine metabolism in cancer: Opportunities and challenges. Trends Pharmacol. Sci. 2024, 45, 395–405. [Google Scholar] [CrossRef]
- Eckert, M.A.; Coscia, F.; Chryplewicz, A.; Chang, J.W.; Hernandez, K.M.; Pan, S.; Tienda, S.M.; Nahotko, D.A.; Li, G.; Blaženović, I.; et al. Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature 2019, 569, 723–728. [Google Scholar] [CrossRef]
- Sanderson, S.M.; Gao, X.; Dai, Z.; Locasale, J.W. Methionine metabolism in health and cancer: A nexus of diet and precision medicine. Nat. Rev. Cancer 2019, 19, 625–637. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Lin, H.; Wang, J.; Fu, J.; Zhu, D.; Xu, W. Inhibition of MAT2A-Related Methionine Metabolism Enhances The Efficacy of Cisplatin on Cisplatin-Resistant Cells in Lung Cancer. Cell J. 2022, 24, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Morehead, L.C.; Garg, S.; Wallis, K.F.; Simoes, C.C.; Siegel, E.R.; Tackett, A.J.; Miousse, I.R. Increased Response to Immune Checkpoint Inhibitors with Dietary Methionine Restriction in a Colorectal Cancer Model. Cancers 2023, 15, 4467. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tan, Y.T.; Chen, Y.X.; Zheng, X.J.; Wang, W.; Liao, K.; Mo, H.Y.; Lin, J.; Yang, W.; Piao, H.L.; et al. Methionine deficiency facilitates antitumour immunity by altering m(6)A methylation of immune checkpoint transcripts. Gut 2023, 72, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Lu, F.; Chang, Z.; Li, J.; Gao, Y.; Zhou, J.; Luo, Y.; Lai, Y.; Cao, S.; Li, X.; et al. Intermittent dietary methionine deprivation facilitates tumoral ferroptosis and synergizes with checkpoint blockade. Nat. Commun. 2023, 14, 4758. [Google Scholar] [CrossRef]
| Methyl-Donor Nutrient | Primary Role in One-Carbon Metabolism | Key Molecular Mechanisms | Representative Molecular Targets/Pathways | Functional Consequences in Cancer Prevention | Functional Consequences in Cancer Progression |
|---|---|---|---|---|---|
| Folate | Supplies one-carbon units for nucleotide synthesis and remethylation of homocysteine to methionine | Maintains SAM availability; regulates DNA synthesis and DNA/histone methylation | DNMTs; LINE-1; PDGF-B; survivin; p16; p53 | Preserves genomic stability; prevents global DNA hypomethylation; suppresses oncogene activation and chromosomal instability | Fuels nucleotide biosynthesis in rapidly dividing cells; may enhance tumor growth and recurrence when preneoplastic or malignant cells are present |
| Vitamin B12 | Cofactor for MS linking folate and methionine cycles | Prevents methyl-folate trap; sustains SAM synthesis; stabilizes methylation capacity | MS; SAM/SAH ratio; p16; p53 | Supports effective remethylation; prevents homocysteine accumulation and epigenetic instability in early carcinogenesis | Excessive supplementation may exacerbate aberrant DNA methylation patterns and promote progression in populations with latent neoplasia |
| Vitamin B6 | Cofactor for SHMT and transsulfuration pathway | Maintains nucleotide integrity; prevents uracil misincorporation; supports redox balance | SHMT; CBS; CSE; ODC1; GOT2; NK cell metabolic pathways | Reduces DNA strand breaks; lowers homocysteine; mitigates oxidative stress and inflammation | Supports metabolic addiction of tumor cells (polyamine synthesis, anaplerosis); depletion in TME impairs anti-tumor immunity |
| Choline/Betaine | Alternative methyl donor via BHMT pathway | Maintains methionine and SAM pools; regulates DNMT activity | BHMT; DNMTs; c-myc; p16; p53 | Prevents global DNA hypomethylation; maintains epigenetic fidelity and lipid homeostasis | Excess availability may support methylation-dependent silencing and lipid-mediated oncogenic signaling |
| Methionine | Direct precursor of SAM | Controls transmethylation flux; regulates epigenetic and redox pathways | MAT2A; PRMT5; histone methylation marks (H3K4me3, H3K9me3); cGAS–STING | Essential for normal methylation and genomic maintenance at physiological levels | Tumor cells exhibit “methionine addiction”; restriction disrupts epigenetic programs, induces metabolic stress and ferroptosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Pan, D.; Wang, S.; Sun, G. The Double-Edged Nature of Methyl Donors in Cancer Development from Prevention to Progression. Int. J. Mol. Sci. 2026, 27, 323. https://doi.org/10.3390/ijms27010323
Pan D, Wang S, Sun G. The Double-Edged Nature of Methyl Donors in Cancer Development from Prevention to Progression. International Journal of Molecular Sciences. 2026; 27(1):323. https://doi.org/10.3390/ijms27010323
Chicago/Turabian StylePan, Da, Shaokang Wang, and Guiju Sun. 2026. "The Double-Edged Nature of Methyl Donors in Cancer Development from Prevention to Progression" International Journal of Molecular Sciences 27, no. 1: 323. https://doi.org/10.3390/ijms27010323
APA StylePan, D., Wang, S., & Sun, G. (2026). The Double-Edged Nature of Methyl Donors in Cancer Development from Prevention to Progression. International Journal of Molecular Sciences, 27(1), 323. https://doi.org/10.3390/ijms27010323








