Effect of Suberoylanilide Hydroxamic Acid and Phytosulfokine-Alpha on Successful Plant Regeneration from Embryogenic Callus-Derived Protoplasts of Garlic (Allium sativum L.)
Abstract
1. Introduction
2. Results
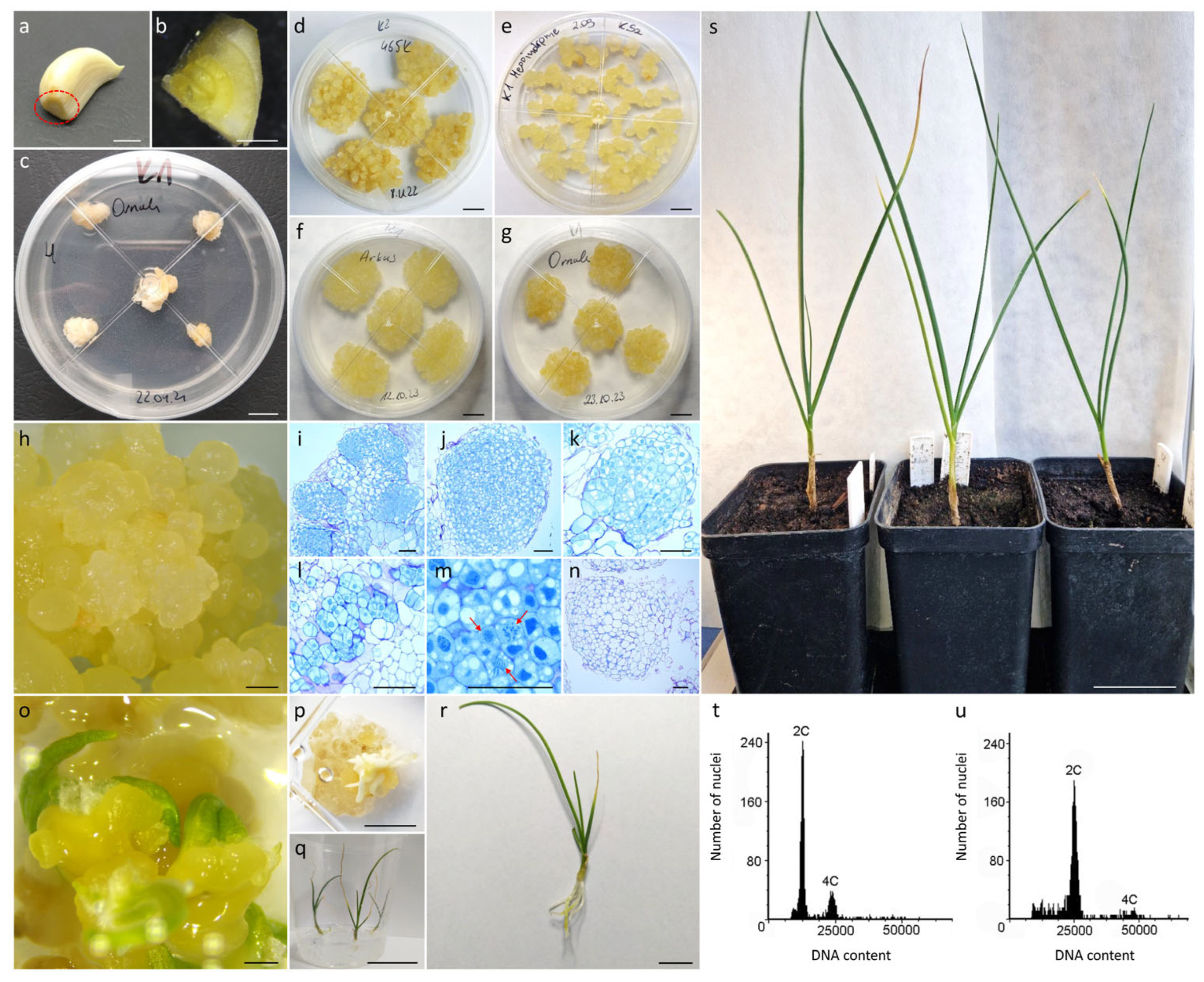
2.1. Induction and Culture of Clove-Derived Callus
2.2. Histological Analysis of Clove-Derived Callus and Plant Regeneration
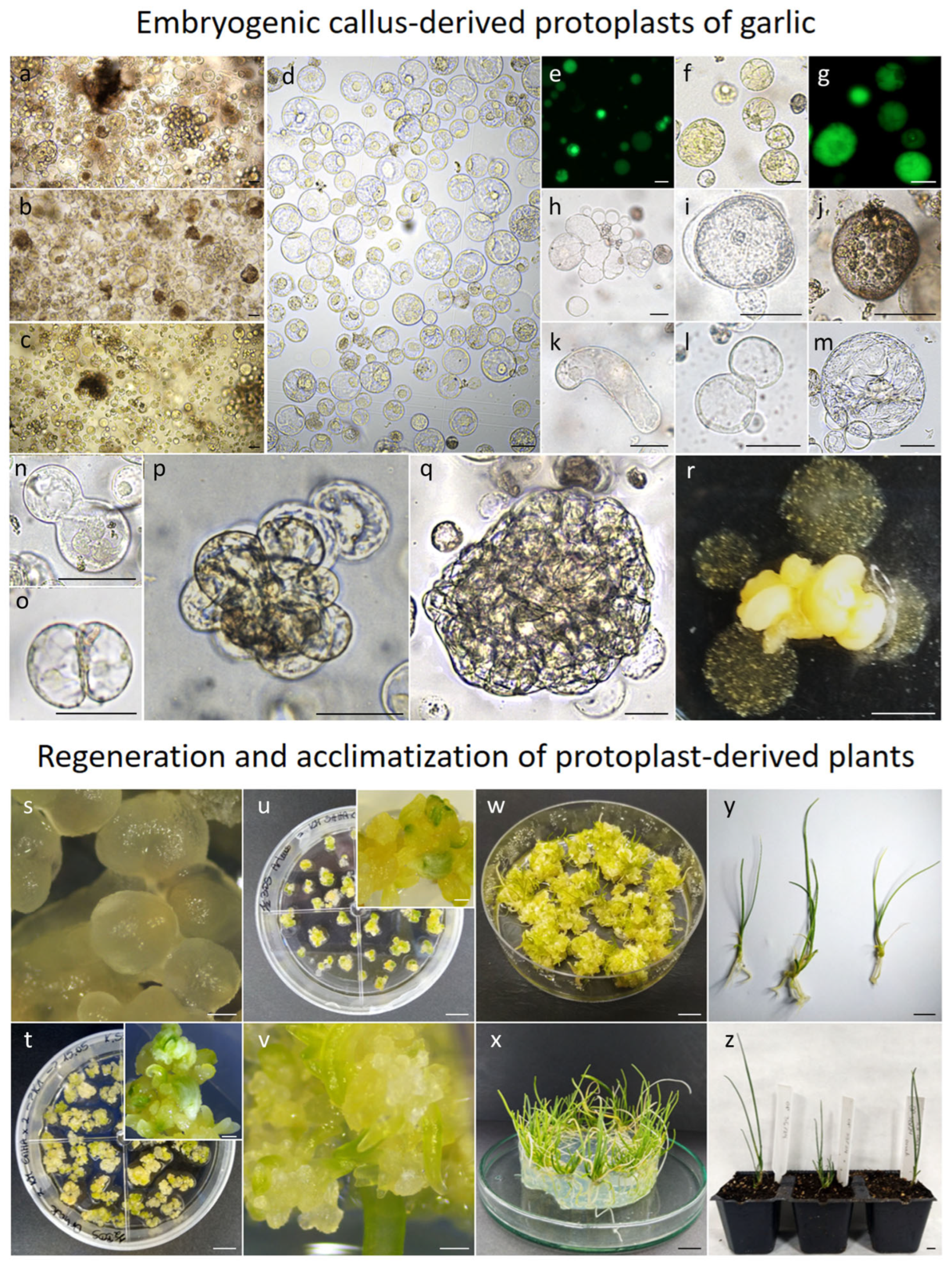
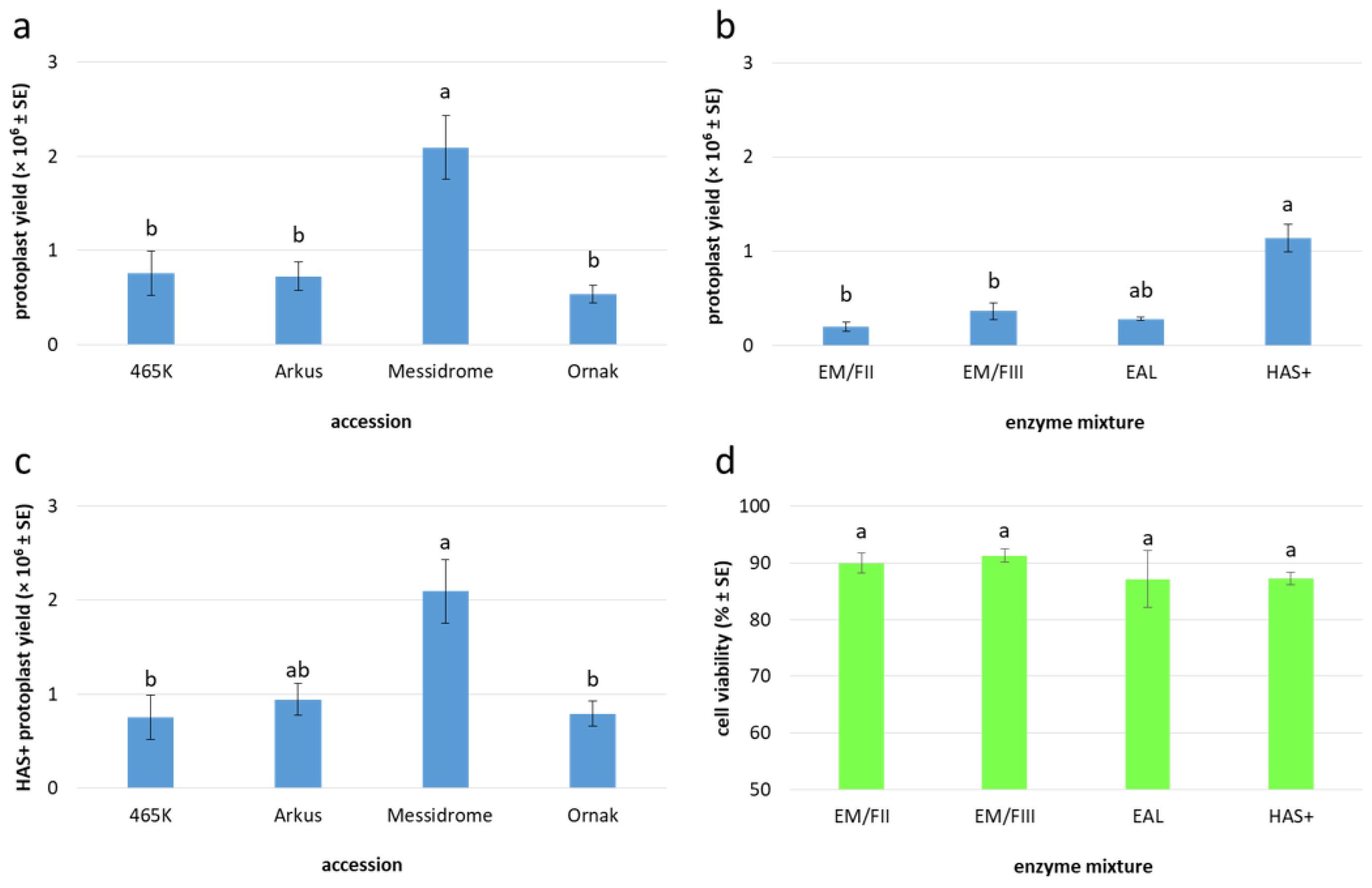
2.3. Yield and Quality of Embryogenic Callus-Derived Protoplasts
2.4. Protoplast Development, Cell Colony and Callus Formation
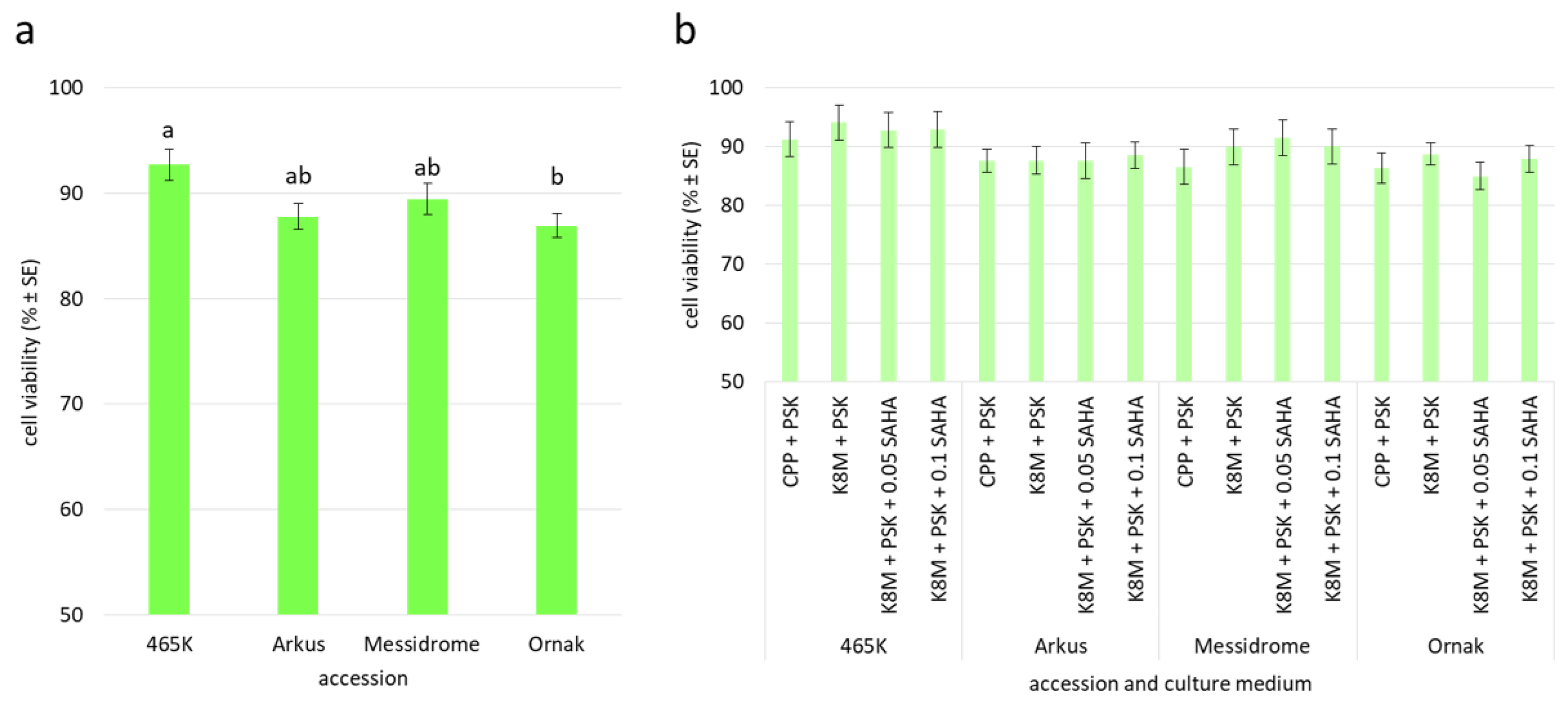
2.5. Plant Regeneration and Ploidy Status of Embryogenic Callus Protoplast-Derived Plants
3. Discussion
3.1. Establishment of an Efficient Embryogenic Callus Induction and Somatic Embryogenesis Protocol for Garlic Regeneration
3.2. Optimized Protocol for Garlic Protoplast Regeneration: Insights into Embryogenic Callus, Culture Conditions, and SAHA-Mediated Epigenetic Enhancement
4. Materials and Methods
4.1. Plant Materials
4.2. Induction of Callogenesis and Establishment of Stable Callus Cultures
4.3. Histological Analysis of Callus and Plant Regeneration from Embryogenic Callus Cultures
4.4. Protoplast Isolation from Embryogenic Callus
4.5. Culture of Callus-Derived Protoplasts and Production of Callus Protoplast-Derived Plants
4.6. Ploidy Analysis of In Vitro Regenerated Plants
4.7. Data Collection and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| BAP | 6-benzylaminopurine |
| B5 | medium base according to Gamborg et al. [57] |
| FM | fresh mass |
| HDACi | histone deacetylase inhibitors |
| IBA | indole-3-butyric acid |
| KM | medium base according to Kao and Michalyuk [52] |
| MES | 2-(N-morpholino)ethanesulfonic acid |
| MS | medium base according to Murashige and Skoog [55] |
| NAA | α-naphthaleneacetic acid |
| PEM | proembryogenic mass |
| PGRs | plant growth regulators |
| PSK | phytosulfokine |
| SAHA | suberoylanilide hydroxamic acid |
| SE | somatic embryos |
| TSA | trichostatin A |
References
- Tiwari, D.; Gautam, A.; Kumar, R.; Sachan, S. Effect of different doses of nitrogen and sulphur on growth and yield of garlic. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 1601–1610. [Google Scholar] [CrossRef]
- Qaemifar, N.; Borji, H.; Adhami, G. The antiparasitic properties of Allium sativum: Can it be used as a complementary treatment for echinococcosis? J. Lab Anim. Res. 2023, 2, 1–5. [Google Scholar] [CrossRef]
- Scotton, D.C.; Benedito, V.A.; Molfetta, J.B.d.; Rodrigues, B.I.F.; Tulmann-Neto, A.; Figueira, A. response of root explants to in vitro cultivation of marketable garlic cultivars. Hortic. Bras. 2013, 31, 80–85. [Google Scholar] [CrossRef]
- Ayabe, M.; Sumi, S. Establishment of a novel tissue culture method, stem-disc culture, and its practical application to micropropagation of garlic (Allium sativum L.). Plant Cell Rep. 1998, 17, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, M.; Sumi, S. A Novel and efficient tissue culture method—“Stem-disc dome culture”—For producing virus-free garlic (Allium sativum L.). Plant Cell Rep. 2001, 20, 503–507. [Google Scholar] [CrossRef]
- Taşkın, H.; Baktemur, G.; Kurul, M.; Büyükalaca, S. Use of tissue culture techniques for producing virus-free plant in garlic and their identification through Real-Time PCR. Sci. World J. 2013, 2013, 781282. [Google Scholar] [CrossRef] [PubMed]
- Robledo-Paz, A.; Villalobos-Arámbula, V.M.; Jofre-Garfias, A.E. Efficient plant regeneration of garlic (Allium sativum L.) by root-tip culture. In Vitro Cell. Dev. Biol.-Plant 2000, 36, 416–419. [Google Scholar] [CrossRef]
- Kereša, S.; Kurtović, K.; Ban, S.G.; Vončina, D.; Jerčić, I.H.; Bolarić, S.; Lazarević, B.; Godena, S.; Ban, D.; Mihovilović, A.B. Production of virus-free garlic plants through somatic embryogenesis. Agronomy 2021, 11, 876. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.H.; Kim, Y.K.; Park, N.I.; Park, S.U. Plant regeneration of garlic (Allium sativum L.) via somatic embryogenesis. Sci. Res. Essays 2009, 4, 1569–1574. [Google Scholar]
- Muhammad, S.H.; Wada, T.; Hattori, K. Efficient plant regeneration in garlic through somatic embryogenesis from root tip explants. Plant Prod. Sci. 1998, 1, 216–222. [Google Scholar]
- Ayabe, M.; Taniguchi, K.; Sumi, S. Regeneration of whole plants from protoplasts isolated from tissue-cultured shoot primordia of garlic (Allium sativum L.). Plant Cell Rep. 1995, 15, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Robledo-Paz, A.; Tovar-Soto, H.M. Biotechnological tools for garlic propagation and improvement. In Innovations in Biotechnology; Agbo, E., Ed.; Books on Demand: Hamburg, Germany, 2012; pp. 31–56. [Google Scholar]
- Eeckhaut, T.; Lakshmanan, P.S.; Deryckere, D.; Van Bockstaele, E.; Van Huylenbroeck, J. Progress in plant protoplast research. Planta 2013, 238, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.M.; Bargmann, B.O.R. Protoplast regeneration and its use in New Plant Breeding Technologies. Front. Genome Ed. 2021, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Ranaware, A.S.; Kunchge, N.S.; Lele, S.S.; Ochatt, S.J. Protoplast technology and somatic hybridisation in the family Apiaceae. Plants 2023, 12, 1060. [Google Scholar] [CrossRef]
- Hasegawa, H.; Sato, M.; Suzuki, M. Efficient plant regeneration from protoplasts isolated from long-term, shoot primordia-derived calluses of garlic (Allium sativum). J. Plant Physiol. 2002, 159, 449–452. [Google Scholar] [CrossRef]
- Metwally, E.I.; El-Denary, M.E.; Dewir, Y.H. Influences of explant type and enzyme incubation on isolated protoplast density and viability in two garlic cultivars. Pak. J. Bot. 2014, 46, 673–677. [Google Scholar]
- Yamashita, K.; Hisatsune, Y.; Sakamoto, T.; Ishizuka, K.; Tashiro, Y. Chromosome and cytoplasm analyses of somatic hybrids between onion (Allium cepa L.) and garlic (A. sativum L.). Euphytica 2002, 125, 163–167. [Google Scholar] [CrossRef]
- Davey, M.R.; Anthony, P.; Patel, D.; Power, J.B. Plant protoplasts: Isolation, culture and plant regeneration. In Plant Cell Culture: Essential Methods; Davey, M.R., Anthony, P., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 153–173. [Google Scholar]
- Panda, D.; Karmakar, S.; Dash, M.; Tripathy, S.K.; Das, P.; Banerjee, S.; Qi, Y.; Samantaray, S.; Mohapatra, P.K.; Baig, M.J.; et al. Optimized protoplast isolation and transfection with a breakpoint: Accelerating Cas9/sgRNA cleavage efficiency validation in monocot and dicot. Abiotech 2024, 5, 151–168. [Google Scholar] [CrossRef]
- He, X.-Y.; Xu, L.-J.; Xu, X.-S.; Yi, D.-D.; Hou, S.-L.; Yuan, D.-Y.; Xiao, S.-X. Embryogenic callus induction, proliferation, protoplast isolation, and PEG induced fusion in Camellia oleifera. Plant Cell Tiss. Organ. Cult. 2024, 157, 75. [Google Scholar] [CrossRef]
- Monthony, A.S.; Jones, A.M.P. Enhancing protoplast isolation and early cell division from Cannabis sativa callus cultures via phenylpropanoid inhibition. Plants 2024, 13, 130. [Google Scholar] [CrossRef]
- Kiełkowska, A.; Adamus, A. Exogenously applied polyamines reduce reactive oxygen species, enhancing cell division and the shoot regeneration from Brassica oleracea L. var. capitata protoplasts. Agronomy 2021, 11, 735. [Google Scholar] [CrossRef]
- Zaranek, M.; Pérez-Pérez, R.; Milewska-Hendel, A.; Betekhtin, A.; Grzebelus, E. Promotive effect of phytosulfokine—Peptide growth factor—On protoplast cultures development in Fagopyrum tataricum (L.) Gaertn. BMC Plant Biol. 2023, 23, 385. [Google Scholar] [CrossRef]
- Jones, A.M.P.; Shukla, M.R.; Biswas, G.C.G.; Saxena, P.K. Protoplast-to-plant regeneration of American elm (Ulmus americana). Protoplasma 2015, 252, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, R.; Pinski, A.; Zaranek, M.; Beckmann, M.; Mur, L.A.J.; Nowak, K.; Rojek-Jelonek, M.; Kostecka-Gugała, A.; Petryszak, P.; Grzebelus, E.; et al. Effect of potent inhibitors of phenylalanine ammonia-lyase and PVP on in vitro morphogenesis of Fagopyrum tataricum. BMC Plant Biol. 2025, 25, 469, Erratum in BMC Plant Biol. 2025, 25, 702.. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, Y.; Takagi, L.; Sakagami, Y. Phytosulfokine-alpha, a sulfated pentapeptide, stimulates the proliferation of rice cells by means of specific high- and low-affinity binding sites. Proc. Natl. Acad. Sci. USA 1997, 94, 13357–13362. [Google Scholar] [CrossRef]
- Maćkowska, K.; Jarosz, A.; Grzebelus, E. Plant regeneration from leaf-derived protoplasts within the Daucus genus: Effect of different conditions in alginate embedding and phytosulfokine application. Plant Cell Tiss. Organ. Cult. 2014, 117, 241–252. [Google Scholar] [CrossRef]
- Kiełkowska, A.; Adamus, A. Peptide growth factor phytosulfokine-α stimulates cell divisions and enhances regeneration from B. oleracea var. capitata L. protoplast culture. J. Plant Growth Regul. 2019, 38, 931–944. [Google Scholar] [CrossRef]
- Stelmach, K.; Grzebelus, E. Plant regeneration from protoplasts of Pastinaca sativa L. via somatic embryogenesis. Plant Cell Tissue Organ. Cult. 2023, 153, 205–217. [Google Scholar] [CrossRef]
- Grzebelus, E.; Szklarczyk, M.; Greń, J.; Śniegowska, K.; Jopek, M.; Kacińska, I.; Mrozek, K. Phytosulfokine stimulates cell divisions in sugar beet (Beta vulgaris L.) mesophyll protoplast cultures. Plant Growth Regul. 2012, 67, 93–100. [Google Scholar] [CrossRef]
- Vogrinčič, V.; Kastelec, D.; Murovec, J. Phytosulfokine alpha enhances regeneration of transformed and untransformed protoplasts of Brassica oleracea. Front. Plant Sci. 2024, 15, 1379618. [Google Scholar] [CrossRef]
- Joo, S.J.; Choi, S.H.; Jie, E.Y.; Lee, O.R.; Kim, S.W. Phytosulfokine promotes cell division in protoplast culture and adventitious shoot formation in protoplast-derived calluses of Nicotiana benthamiana. Plant Biotechnol. Rep. 2022, 16, 633–643. [Google Scholar] [CrossRef]
- Lee, H.-S.; Han, J.-E.; Bae, E.-K.; Jie, E.Y.; Kim, S.W.; Kwon, H.J.; Lee, H.S.; Yeon, S.-H.; Murthy, H.N.; Park, S.-Y. Response surface methodology mediated optimization of phytosulfokine and plant growth regulators for enhanced protoplast division, callus induction, and somatic embryogenesis in Angelica gigas Nakai. BMC Plant Biol. 2024, 24, 527. [Google Scholar] [CrossRef] [PubMed]
- Ochatt, S.; Conreux, C.; Moussa Mcolo, R.; Despierre, G.; Magnin-Robert, J.-B.; Raffiot, B. Phytosulfokine-alpha, an enhancer of in vitro regeneration competence in recalcitrant legumes. Plant Cell Tiss. Organ. Cult. 2018, 135, 189–201. [Google Scholar] [CrossRef]
- Roubelakis-Angelakis, K.A. An assessment of possible factors contributing to recalcitrance of plant protoplasts. In Morphogenesis in Plants: Molecular Approaches; Roubelakis-Angelakis, K.A., Van Thanh, K.T., Eds.; Springer: Boston, MA, USA, 1993; pp. 201–219. [Google Scholar]
- Pasternak, T.; Lystvan, K.; Betekhtin, A.; Hasterok, R. From single cell to plants: Mesophyll protoplasts as a versatile system for investigating plant cell reprogramming. Int. J. Mol. Sci. 2020, 21, 4195. [Google Scholar] [CrossRef]
- Zhao, J.; Morozova, N.; Williams, L.; Libs, L.; Avivi, Y.; Grafi, G. Two phases of chromatin decondensation during dedifferentiation of plant cells: Distinction between competence for cell fate switch and a commitment for S phase. J. Biol. Chem. 2001, 276, 22772–22778. [Google Scholar] [CrossRef]
- Moricová, P.; Ondřej, V.; Navrátilová, B.; Luhová, L. Changes of DNA methylation and hydroxymethylation in plant protoplast cultures. Acta Biochim. Pol. 2013, 60, 33–36. [Google Scholar] [CrossRef]
- Grzybkowska, D.; Morończyk, J.; Wójcikowska, B.; Gaj, M.D. Azacitidine (5-AzaC)-treatment and mutations in DNA methylase genes affect embryogenic response and expression of the genes that are involved in somatic embryogenesis in Arabidopsis. Plant Growth Regul. 2018, 85, 243–256. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Gao, Y.; Jiang, X.; Zhang, M.; Wu, H.; Liu, Z.; Feng, H. Effects of histone deacetylase inhibitors on microspore embryogenesis and plant regeneration in pakchoi (Brassica rapa ssp. chinensis L.). Sci. Hortic. 2016, 209, 61–66. [Google Scholar] [CrossRef]
- Nowicka, A.; Tokarz, B.; Zwyrtková, J.; Dvořák Tomaštíková, E.; Procházková, K.; Ercan, U.; Finke, A.; Rozhon, W.; Poppenberger, B.; Otmar, M.; et al. Comparative analysis of epigenetic inhibitors reveals different degrees of interference with transcriptional gene silencing and induction of DNA damage. Plant J. 2020, 102, 68–84. [Google Scholar] [CrossRef]
- Ondřej, V.; Kitner, M.; Doležalová, I.; Nádvorník, P.; Navrátilová, B.; Lebeda, A. Chromatin structural rearrangement during dedifferentiation of protoplasts of Cucumis sativus L. Mol. Cells 2009, 27, 443–448. [Google Scholar] [CrossRef]
- Cápal, P.; Ondřej, V. Expression and epigenetic profile of protoplast cultures (Cucumis sativus L.). In Vitro Cell. Dev. Biol. Plant 2014, 50, 789–794. [Google Scholar] [CrossRef]
- Grafi, G. Epigenetics in plant development and response to stress. BBA Gene Regul. Mech. 2011, 1809, 351–352. [Google Scholar] [CrossRef] [PubMed]
- El-Tantawy, A.-A.; Solís, M.-T.; Risueño, M.C.; Testillano, P.S. Changes in DNA methylation levels and nuclear distribution patterns after microspore reprogramming to embryogenesis in barley. Cytogenet. Genome Res. 2014, 143, 200–208. [Google Scholar] [CrossRef]
- De-la-Peña, C.; Nic-Can, G.I.; Galaz-Ávalos, R.M.; Avilez-Montalvo, R.; Loyola-Vargas, V.M. The role of chromatin modifications in somatic embryogenesis in plants. Front. Plant Sci. 2015, 6, 635. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sanz, H.; Moreno-Romero, J.; Solís, M.-T.; Köhler, C.; Risueño, M.C.; Testillano, P.S. Changes in histone methylation and acetylation during microspore reprogramming to embryogenesis occur concomitantly with BnHKMT and BnHAT expression and are associated with cell totipotency, proliferation, and differentiation in Brassica napus. Cytogenet. Genome Res. 2014, 143, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Soriano, M.; Cordewener, J.; Muiño, J.M.; Riksen, T.; Fukuoka, H.; Angenent, G.C.; Boutilier, K. The histone deacetylase inhibitor trichostatin A promotes totipotency in the male gametophyte. Plant Cell 2014, 26, 195–209. [Google Scholar] [CrossRef]
- Liu, C.; Song, G.; Fang, B.; Liu, Z.; Zou, J.; Dong, S.; Du, S.; Ren, J.; Feng, H. Suberoylanilide Hydroxamic Acid induced microspore embryogenesis and promoted plantlet regeneration in ornamental kale (Brassica oleracea var. acephala). Protoplasma 2022, 260, 117–129. [Google Scholar] [CrossRef]
- Wójcikowska, B.; Botor, M.; Morończyk, J.; Wójcik, A.M.; Nodzyński, T.; Karcz, J.; Gaj, M.D. Trichostatin A triggers an embryogenic transition in Arabidopsis explants via an auxin-related pathway. Front. Plant Sci. 2018, 9, 1353. [Google Scholar] [CrossRef]
- Grzebelus, E.; Szklarczyk, M.; Baranski, R. An improved protocol for plant regeneration from leaf- and hypocotyl-derived protoplasts of carrot. Plant Cell Tiss. Organ. Cult. 2012, 109, 101–109. [Google Scholar] [CrossRef]
- Kao, K.N.; Michayluk, M.R. Nutritional requirements for growth of Vicia hajastana cells and protoplasts at a very low population density in liquid media. Planta 1975, 126, 105–110. [Google Scholar] [CrossRef]
- Dunstan, D.I.; Short, K.C. Improved growth of tissue cultures of the onion, Allium cepa. Physiol. Plant. 1977, 41, 70–72. [Google Scholar] [CrossRef]
- Bohanec, B.; Jakše, M. Variations in gynogenic response among long-day onion (Allium cepa L.) accessions. Plant Cell Rep. 1999, 18, 737–742. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Koch, M.; Tanami, Z.; Salomon, R. Improved regeneration of shoots from garlic callus. HortScience 1995, 30, 378. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Luciani, G.F.; Mary, A.K.; Pellegrini, C.; Curvetto, N.R. Effects of explants and growth regulators in garlic callus formation and plant regeneration. Plant Cell Tiss. Organ. Cult. 2006, 87, 139–143. [Google Scholar] [CrossRef]
- Asghar, S.; Ghori, N.; Hyat, F.; Li, Y.; Chen, C. Use of auxin and cytokinin for somatic embryogenesis in plant: A story from competence towards completion. Plant Growth Regul. 2023, 99, 413–428. [Google Scholar] [CrossRef]
- Haider, S.R.; Hossain, M.R.; Rahman, S.; Sultana, S.; Quddus, T.; Chakraborti, M.; Hoque, A.; Shahriar, M.H.; Haque, M.A. In vitro plantlet regeneration of four local garlic (Allium sativum) accessions of Bangladesh. Biotechnol. J. Int. 2015, 8, 1–12. [Google Scholar] [CrossRef]
- Mostafa, H.H.A.; Wang, H.; Song, J.; Li, X. Effects of genotypes and explants on garlic callus production and endogenous hormones. Sci. Rep. 2020, 10, 4867. [Google Scholar] [CrossRef]
- El Abidine Triqui, Z.; Guédira, A.; Chlyah, A.; Chlyah, H.; Souvannavong, V.; Haïcour, R.; Sihachakr, D. Effect of genotype, gelling agent, and auxin on the induction of somatic embryogenesis in sweet potato (Ipomoea batatas Lam.). C. R. Biol. 2008, 331, 198–205. [Google Scholar] [CrossRef]
- Głowacka, K.; Jeżowski, S.; Kaczmarek, Z. The effects of genotype, inflorescence developmental stage and induction medium on callus induction and plant regeneration in two miscanthus species. Plant Cell Tiss. Organ. Cult. 2010, 102, 79–86. [Google Scholar] [CrossRef]
- Hapsoro, D.; Hamiranti, R.; Yusnita, Y. In vitro somatic embryogenesis of superior clones of robusta coffee from Lampung, Indonesia: Effect of genotypes and callus induction media. Biodiversitas 2020, 21, 3811–3817. [Google Scholar] [CrossRef]
- Naing, A.H.; Adedeji, O.S.; Kim, C.K. Protoplast technology in ornamental plants: Current progress and potential applications on genetic improvement. Sci. Hort. 2021, 283, 110043. [Google Scholar] [CrossRef]
- Masani, M.Y.A.; Noll, G.; Parveez, G.K.A.; Sambanthamurthi, R.; Prüfer, D. Regeneration of viable oil palm plants from protoplasts by optimizing media components, growth regulators and cultivation procedures. Plant Sci. 2013, 210, 118–127. [Google Scholar] [CrossRef]
- Horstman, A.; Bemer, M.; Boutilier, K. A transcriptional view on somatic embryogenesis. Regeneration 2017, 4, 201–216. [Google Scholar] [CrossRef]
- Sugimoto, K.; Temman, H.; Kadokura, S.; Matsunaga, S. To regenerate or not to regenerate: Factors that drive plant regeneration. Curr. Opin. Plant Biol. 2019, 47, 138–150. [Google Scholar] [CrossRef]
- Tai, H.H.; Tai, G.C.C.; Beardmore, T. Dynamic histone acetylation of late embryonic genes during seed germination. Plant Mol. Biol. 2005, 59, 909–925. [Google Scholar] [CrossRef]
- Uddenberg, D.; Valladares, S.; Abrahamsson, M.; Sundström, J.F.; Sundås-Larsson, A.; von Arnold, S. Embryogenic potential and expression of embryogenesis-related genes in conifers are affected by treatment with a histone deacetylase inhibitor. Planta 2011, 234, 527–539. [Google Scholar] [CrossRef]
- Menczel, L.; Nagy, F.; Kiss, Z.R.; Maliga, P. Streptomycin resistant and sensitive somatic hybrids of Nicotiana tabacum + Nicotiana knightiana: Correlation of resistance to N. tabacum plastids. Theor. Appl. Genet. 1981, 59, 191–195. [Google Scholar] [CrossRef]
- Dirks, R.; Sidorov, V.; Tulmans, C. A new protoplast culture system in Daucus carota L. and its applications for mutant selection and transformation. Theoret. Appl. Genet. 1996, 93, 809–815. [Google Scholar] [CrossRef]
- Sliwinska, E.; Loureiro, J.; Leitch, I.J.; Šmarda, P.; Bainard, J.; Bureš, P.; Chumová, Z.; Horová, L.; Koutecký, P.; Lučanová, M.; et al. Application-based guidelines for best practices in plant flow cytometry. Cytom. Part A 2022, 101, 749–781. [Google Scholar] [CrossRef]
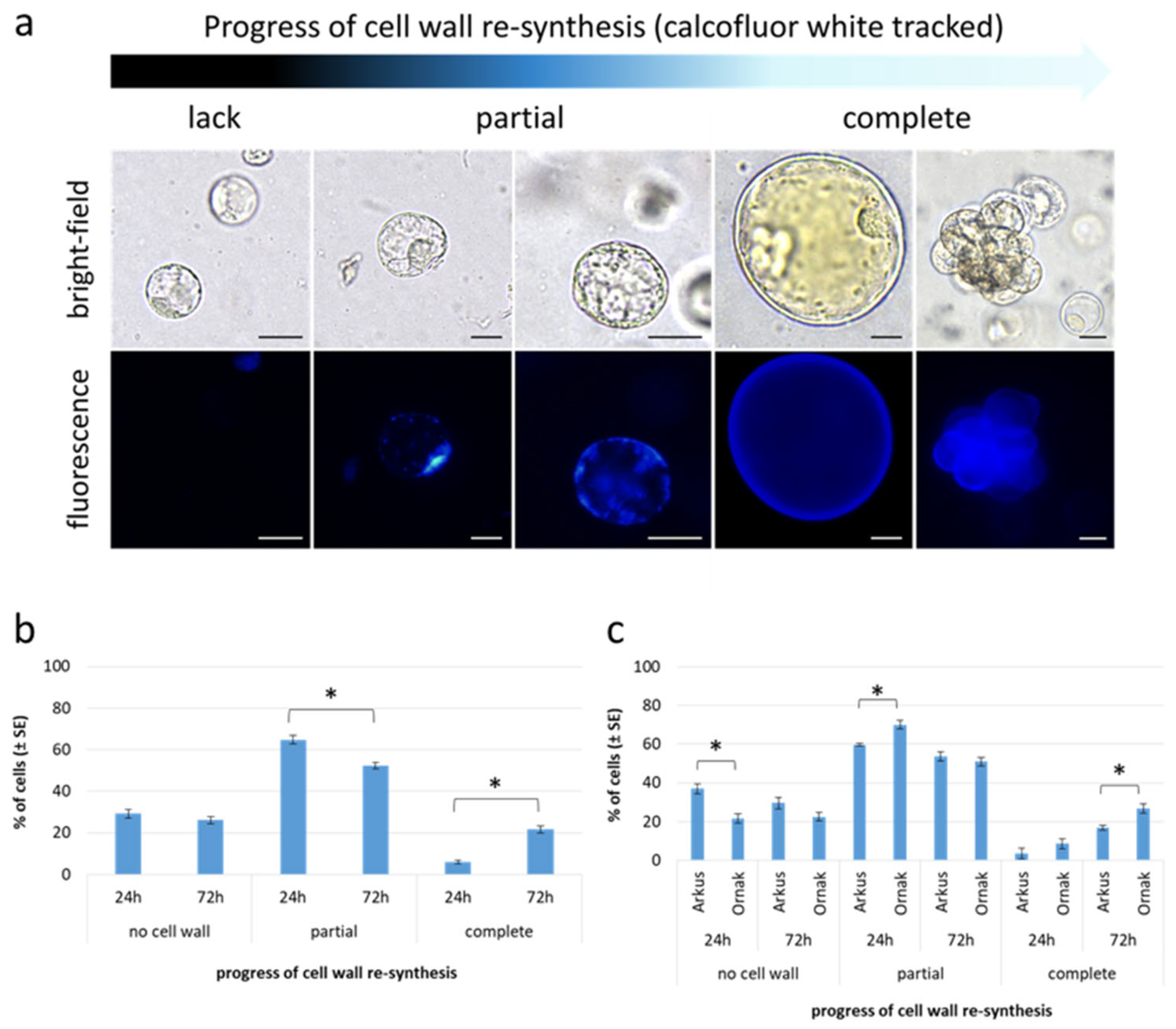
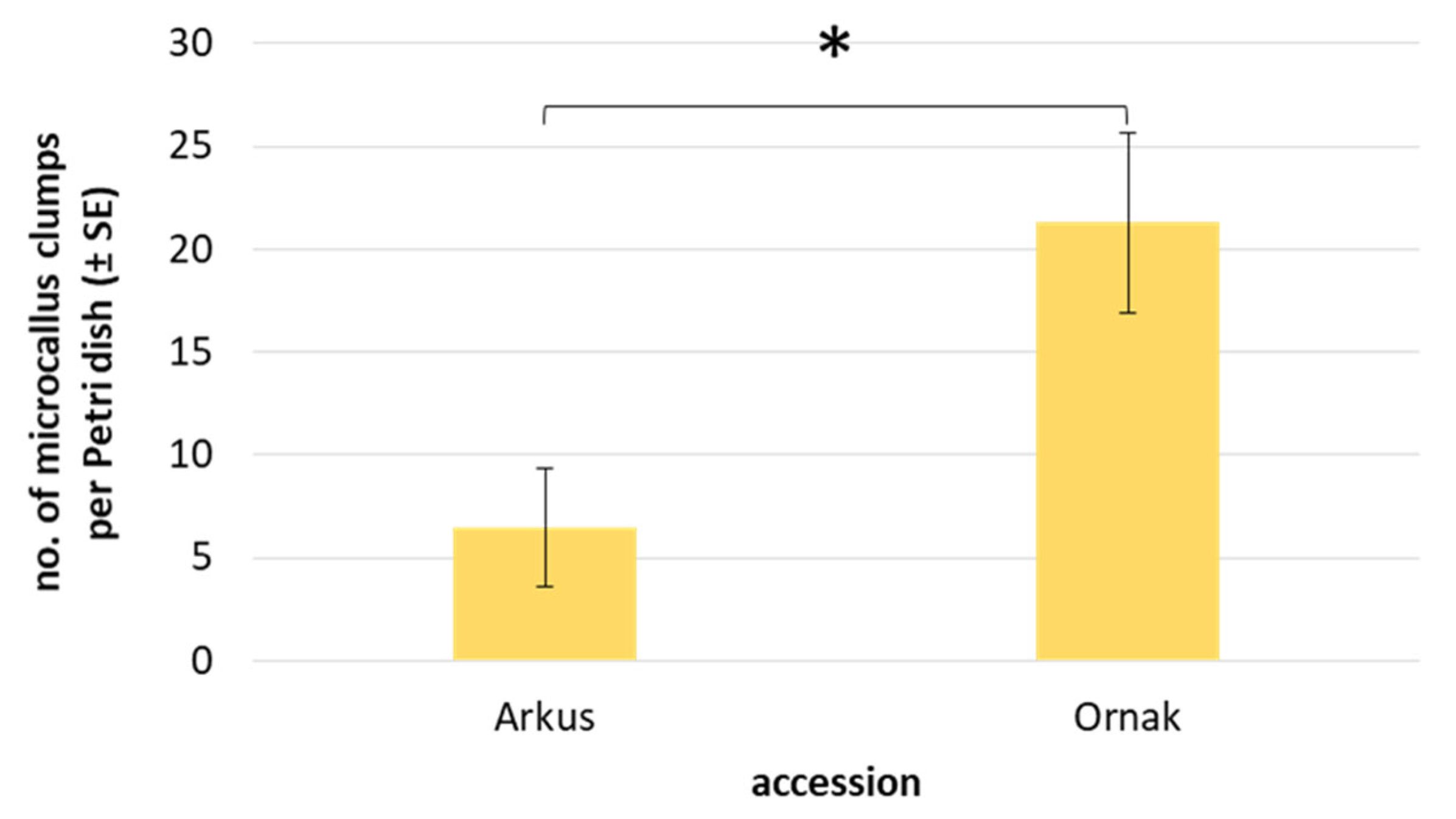
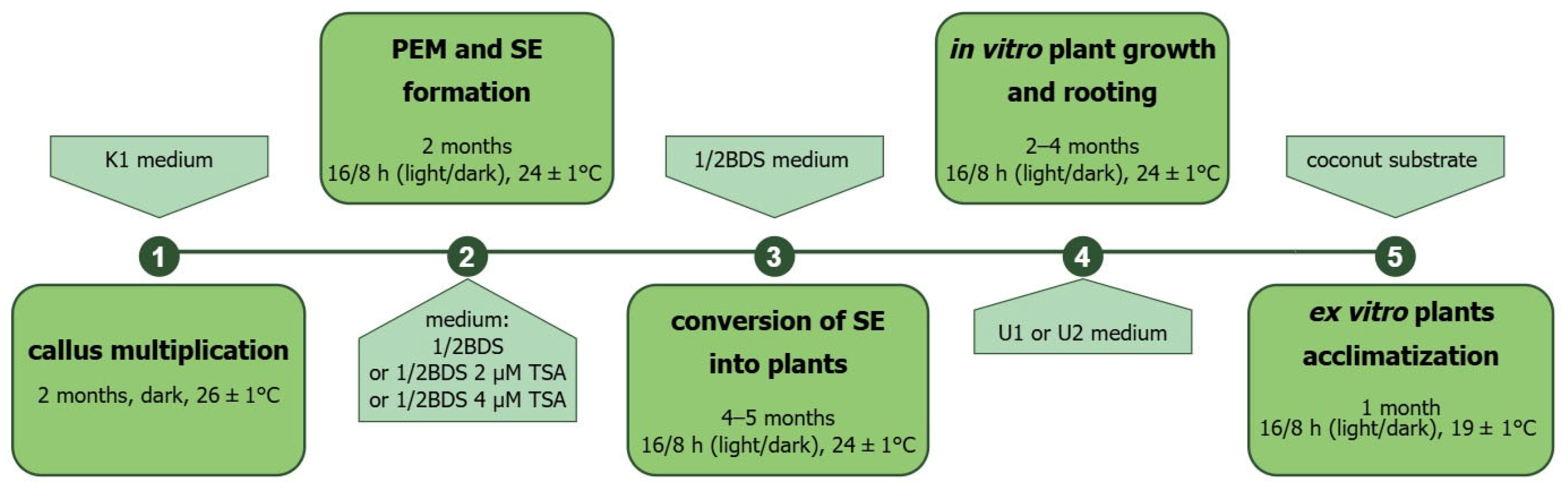
| Accession | Callus Induction Medium | Mean No. of Cultured Explants 1 | Mean No. of Explants Forming Callus ± SE | Structure of Callus (No. of Explants ± SE) | Type of Callus (No. of Explants ± SE) | ||
|---|---|---|---|---|---|---|---|
| Friable | Watery | Embryogenic | Nonembryogenic | ||||
| 465K | K1 | 50 | 40.7 ± 3.5 (81.4%) | 0.0 | 40.7 ± 3.5 | 31.7 ± 10.7 | 9.0 ± 7.5 |
| K2 | 50 | 42.7 ± 4.5 (85.4%) | 42.7 ± 4.5 | 0.0 | 19.0 ± 11.5 | 23.7 ± 9.9 | |
| Arkus | K1 | 50 | 24.3 ± 9.4 (48.7%) | 22.0 ± 10.5 | 2.3 ± 1.5 | 17.3 ± 7.4 | 7.0 ± 2.1 |
| K2 | 50 | 21.3 ± 3.3 (42.7%) | 21.3 ± 3.3 | 0.0 | 2.3 ± 2.3 | 19.0 ± 2.3 | |
| Messidrome | K1 | 50 | 7.7 ± 0.3 (15.3%) | 7.7 ± 0.3 | 0.0 | 0.4 ± 0.3 | 7.3 ± 0.3 |
| K2 | 50 | 13.0 ± 2.1 (26.0%) | 13.0 ± 2.1 | 0.0 | 4.3 ± 1.7 | 8.7 ± 0.7 | |
| Ornak | K1 | 50 | 31.3 ± 6.4 (62.7%) | 19.7 ± 6.7 | 11.7 ± 4.3 | 20.6 ± 10.8 | 10.7 ± 5.6 |
| K2 | 50 | 41.3 ± 2.9 (82.7%) | 41.3 ± 2.9 | 0.0 | 24.0 ± 9.3 | 17.3 ± 6.5 | |
| Mean | – | 50 | 27.8 ± 3.0 (56.1%) | 21.0 ± 3.3 | 6.8 ± 2.8 | 15.0 ± 3.2 | 12.8 ± 2.0 |
| Test Summary | Compared Factors | |||||
|---|---|---|---|---|---|---|
| Accession and No. of Explants Forming Callus | Induction Medium and No. of Explants Forming Callus | Accession and Friable Callus Formation | Induction Medium and Friable Callus Formation | Accession and Embryogenic Callus Formation | Induction Medium and Embryogenic Callus Formation | |
| Total N | 24 | 24 | 24 | 24 | 24 | 24 |
| Test statistics (H) | 16.06 | 0.48 | 4.94 | 10.90 | 5.15 | 1.62 |
| Degree of freedom | 3 | 1 | 3 | 1 | 3 | 1 |
| Asymptotic significance (p) | 0.001 | 0.488 | 0.176 | 0.001 | 0.161 | 0.203 |
| Accession | Enzyme Mixture | Callus Age (Days) | n | Protoplast Yield (×106/g FM) [Mean ± SE] | Protoplast Viability (%) [Mean ± SE] |
|---|---|---|---|---|---|
| 465K | HAS+ | 15–20 | 4 | 0.75 ± 0.24 (b) 1 | 86.25 ± 3.24 (a) |
| Arkus | EM/FII | 13 | 1 | 0.12 | 94.43 |
| EAL | 11–12 | 3 | 0.28 ± 0.02 | 87.18 ± 5.01 | |
| HAS+ | 10–16 | 9 | 0.94 ± 0.17 | 86.80 ± 1.92 | |
| total/mean | 10–16 | 13 | 0.73 ± 0.15 (b) | 87.53 ± 1.76 (a) | |
| Messidrome | HAS+ | 13–17 | 6 | 2.09 ± 0.34 (a) | 88.36 ± 2.85 (a) |
| Ornak | EM/FII | 13–16 | 3 | 0.23 ± 0.06 | 88.87 ± 1.74 |
| EM/FIII | 14–17 | 5 | 0.36 ± 0.08 | 91.37 ± 1.20 | |
| HAS+ | 12–16 | 7 | 0.79 ± 0.14 | 87.61 ± 1.67 | |
| total/mean | 12–17 | 15 | 0.54 ± 0.09 (b) | 89.10 ± 0.89 (a) | |
| Total/Mean | 10–20 | 38 | 0.87 ± 0.12 | 88.69 ± 0.82 |
| Types of Developmental/Degeneration Events | Protoplast Culture Medium/Age of the Culture (Days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CPP + PSK | K8M + PSK | K8M + PSK + 0.05 SAHA | K8M + PSK + 0.1 SAHA | ||||||
| 20 | 30 | 20 | 30 | 20 | 30 | 20 | 30 | ||
| pre-mitotic events | increase in size | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| reorganization of cytoplasm and organelles | ++ | ++ | +++ | + | +++ | ++ | +++ | ++ | |
| unfinished cell divisions | − | + | + | + | + | + | + | + | |
| post-mitotic events | cell colony formation | − | − | − | ++ | + | ++ | + | +++ |
| cell degeneration indicators | plasmolysis | ++ | ++ | + | + | + | + | + | + |
| cell elongation | ++ | +++ | + | +++ | + | +++ | + | ++ | |
| cell fragmentation | + | ++ | ++ | +++ | ++ | ++ | ++ | ++ | |
| cell browning | ++ | +++ | + | ++ | + | ++ | + | ++ | |
| Protoplast Culture Medium | Accession | |||
|---|---|---|---|---|
| 465K | Arkus | Messidrome | Ornak | |
| CPP + PSK | − | − | −/+ | −/+ |
| K8M + PSK | − | + | + | + |
| K8M + PSK + 0.05 SAHA | − | ++ | ++ | +++ |
| K8M + PSK + 0.1 SAHA | − | ++ | ++ | +++ |
| Protoplast Culture Medium | Regeneration → Rooting Media | n | No. of Obtained Plants | |
|---|---|---|---|---|
| Total | Mean/Petri Dish 1 | |||
| K8M + PSK + 0.05 SAHA | ½ BDS → U1 | 15 | 844 | 53.6 ± 6.8 (a) |
| K8M + PSK + 0.05 SAHA | ½ BDS + 2 µM TSA → ½ BDS → U1 | 3 | 20 | 6.8 ± 2.9 (b) |
| K8M + PSK + 0.05 SAHA | ½ BDS + 4 µM TSA → ½ BDS → U1 | 3 | 45 | 15.0 ± 1.7 (b) |
| K8M + PSK + 0.05 SAHA | ½ BDS → U2 | 5 | 196 | 39.2 ± 4.2 (ab) |
| K8M + PSK + 0.1 SAHA | ½ BDS → U1 | 1 | 14 | - |
| Protoplast Culture Medium | Regeneration → Rooting Media | Plant Acclimatization (No.) | Ploidy Level (No.) | ||||
|---|---|---|---|---|---|---|---|
| Planted | Acclimatized | Analyzed | 2x | 4x | 2x–4x | ||
| K8M + PSK + 0.05 SAHA | ½ BDS → U1 | 183 | 128 (70.0%) | 36 | 35 | 0 | 1 |
| K8M + PSK + 0.05 SAHA | ½ BDS + 2 µM TSA → ½ BDS → U1 | 19 | 6 (31.6%) | 6 | 5 | 1 | 0 |
| K8M + PSK + 0.05 SAHA | ½ BDS + 4 µM TSA → ½ BDS → U1 | 45 | 22 (49.0%) | 22 | 1 | 21 | 0 |
| K8M + PSK + 0.05 SAHA | ½ BDS → U2 | 0.0 1 | - | - | - | - | - |
| K8M + PSK + 0.1 SAHA | ½ BDS → U1 | 14 | 12 (85.7%) | 12 | 10 | 0 | 2 |
| Accession Name | Accession Status/Country of Origin | Bulb Source 1 | Biological and Functional Characteristics | Experiment Type |
|---|---|---|---|---|
| 465K | gene bank accession/UA | NIHR, PL | early harvest, no other data available | callus induction protoplast cultures |
| Arkus | cultivar/PL | PlantiCo, PL | medium-early harvest, hardneck winter-hardy cultivar; recommended for direct consumption and processing | callus induction protoplast cultures |
| Messidrome | cultivar/FRA | Benex, PL | early harvest, softneck winter-hardy cultivar; recommended for direct consumption | callus induction protoplast cultures |
| Ornak | cultivar/PL | PlantiCo, PL | medium-late harvest, hardneck winter-hardy garlic cultivar; recommended for direct consumption and processing | callus induction histological analysis protoplast cultures |
| Solution/Medium Name | Solution/Medium Composition | Application | Storage Conditions |
|---|---|---|---|
| K1 (based on [54] BDS formula) | Gamborg B5 micro- and macroelements with vitamins ([58]; Duchefa Biochemie), 30 g L−1 sucrose (POCH, Gliwice, Poland), 320 mg L−1 NH4NO3 (POCH), 230 mg L−1 NH4H2PO4 (POCH); 2.0 mg L−1 2,4-dichlorophenoxyacetic acid (2,4-D; Sigma-Merck); 0.6% (w/v) plant agar (Duchefa); pH 5.8; autoclaved | callus induction and multiplication | room temperature (RT) |
| K2 (based on [54] BDS formula) | Gamborg B5 micro- and macroelements with vitamins ([58]; Duchefa), 30 g L−1 sucrose (POCH), 320 mg L−1 NH4NO3 (POCH), 230 mg L−1 NH4H2PO4 (POCH); 1.0 mg L−1 2,4-D (Sigma, St. Louis, MO, USA); 2.0 mg L−1 6-benzylaminopurine (BAP; Sigma); 0.6% (w/v) plant agar (Duchefa); pH 5.8; autoclaved | callus induction and multiplication | RT |
| ½ BDS ([54]) | 0.5× Gamborg B5 micro- and macroelements with vitamins ([58]; Duchefa); 30 g L−1 sucrose (POCH); 160 mg L−1 NH4NO3 (POCH), 115 mg L−1 NH4H2PO4 (POCH); 0.6% (w/v) plant agar (Duchefa); pH 5.8; autoclaved | plant regeneration | RT |
| PSII | 0.5 M mannitol (Sigma); pH 5.6; autoclaved | plasmolysis | RT |
| W5 [72] | 154 mM sodium chloride (POCH), 125 mM calcium chloride dihydrate (POCH), 5 mM potassium chloride (POCH), 5 mM glucose (POCH); pH 5.8; autoclaved | protoplast purification | RT |
| K8M medium [53] | KM micro- and macroelements ([53]; Duchefa), 100 mg L−1 myo-inositol (Duchefa), 0.01 mg L−1 retinyl acetate (Sigma), 1 mg L−1 thiamine (Sigma), 0.2 mg L−1 riboflavin (Sigma), 1 mg L−1 nicotinamide (Sigma), 1 mg L−1 D-calcium pantothenate (Duchefa), 1 mg L−1 pyridoxine (Sigma), 0.01 mg L−1 biotin (Duchefa), 0.4 mg L−1 folic acid (Duchefa), 0.02 mg L−1 cyanocobalamin (Sigma), 2 mg L−1 ascorbic acid (Duchefa), 0.01 mg L−1 cholecalciferol, 20 mg L−1 sodium pyruvate (Sigma), 40 mg L−1 citric acid (Sigma), 40 mg L−1 malic acid (Sigma), 40 mg L−1 fumaric acid (Sigma), 1 mg L−1 choline chloride (Sigma), 0.02 mg L−1 p-aminobenzoic acid (Sigma), 68.4 g L−1 glucose (POCH), 250 mg L−1 sucrose (POCH), 250 mg L−1 fructose (Duchefa), 250 mg L−1 ribose (Duchefa), 250 mg L−1 xylose (Duchefa), 250 mg L−1 mannose (Duchefa), 250 mg L−1 rhamnose (Duchefa), 250 mg L−1 cellobiose (Sigma), 250 mg L−1 sorbitol (POCH), 250 mg L−1 mannitol (Sigma), 0.6 mg L−1 L-alanine, 0.1 mg L−1 L-arginine-HCl, 0.1 mg L−1 L-asparagine, 0.1 mg L−1 aspartic acid, 0.2 mg L−1 L-cysteine, 0.1 mg L−1 L-cystine, 0.6 mg L−1 L-glutamic acid, 5.6 mg L−1 L-glutamine, 0.1 mg L−1 L-glycine, 0.1 mg L−1 L-histidine hydrochloride, 0.1 mg L−1 4-hydroxyproline, 0.1 mg L−1 L-isoleucine, 0.1 mg L−1 L-leucine, 0.1 mg L−1 L-lysine hydrochloride, 0.1 mg L−1 L-methionine, 0.1 mg L−1 L-phenylalanine, 0.1 mg L−1 L-proline, 0.1 mg L−1 L-serine, 0.1 mg L−1 L-threonine, 0.1 mg L−1 L-tryptophan, 0.1 mg L−1 L-tyrosine, 0.1 mg L−1 L-valine (all amino acids provided by Sigma), 0.1 mg L−1 adenine (Sigma), 0.3 mg L−1 guanine (Sigma), 0.3 mg L−1 thymine (Sigma), 0.3 mg L−1 uracil (Sigma), 0.015 mg L−1 hypoxanthine (Sigma), 0.03 mg L−1 xanthine (Sigma), 0.1 mg L−1 2,4-D (Sigma), 1 mg L−1 1-naphthaleneacetic acid (NAA; Sigma), 0.2 mg L−1 zeatin (Sigma), 250 mg L−1 N-Z-amine (Sigma), 20 mL coconut water (Sigma); pH 5.6; filtered (0.22 μm membrane; Sterivex-GP, Millipore-Merck, Darmstadt, Germany) | protoplast culture | 4 °C, dark |
| CPP medium [73] | KM micro- and macroelements ([53]; Duchefa), vitamins ([58]; Duchefa), 20 mg L−1 sodium pyruvate (Sigma), 40 mg L−1 citric acid (Sigma), 40 mg L−1 malic acid (Sigma), 40 mg L−1 fumaric acid (Sigma), 0.4 M glucose (POCH), 250 mg L−1 N-Z-amine (Sigma), 0.1 mg L−1 2,4-D (Sigma), and 0.2 mg L−1 zeatin (Sigma); pH 5.6; filtered (0.22 μm membrane; Sterivex-GP, Millipore) | protoplast culture | 4 °C, dark |
| U1 medium (based on [55] formula with modifications) | Gamborg B5 micro- and macroelements with vitamins ([58]; Duchefa), 20 g L−1 sucrose (POCH), 15 g L−1 maltose (Duchefa), 400 mg L−1 myo-inositol (Duchefa), 200 mg L−1 proline (Duchefa), 100 mg L−1 2-(N-morpholino)ethanesulfonic acid (MES buffer; Sigma), 320 mg L−1 NH4NO3 (POCH), 230 mg L−1 NH4H2PO4 (POCH), 0.5 mg L−1 indole-3-butyric acid (IBA; Duchefa), 0.28% (w/v) gerlite (Duchefa); pH 5.6; autoclaved | plant rooting and shoot growth | RT |
| U2 medium | MS micro- and macroelements with vitamins ([56]; Duchefa), 20 g L−1 sucrose (POCH), 0.5 mg L−1 NAA (Duchefa), 0.6% (w/v) plant agar (Duchefa), pH 5.8; autoclaved | plant rooting and shoot growth | RT |
| Component | Enzyme Mixture Name | |||
|---|---|---|---|---|
| EM/FII | EM/FIII | EAL | HAS+ | |
| Cellulase Onozuka R10 (Duchefa) | 1% | 1% | 1% | 2% |
| Pectolyase Y-23 (Duchefa) | - | - | 0.04% | 0.2% |
| Macerozyme R-10 (Duchefa) | 0.6% | 0.6% | 0.4% | - |
| Driselase (Sigma-Merk) | 0.15% | 0.3% | 0.09% | - |
| Mannitol (Sigma-Merk) | 0.6 M | 0.6 M | 0.6 M | 0.55 M |
| MES buffer (Sigma-Merk) | 20 mM | 20 mM | 20 mM | 5 mM |
| MgCl2 × 6H2O (POCH) | 5 mM | 5 mM | 5 mM | 5 mM |
| N-Z-amine (Sigma-Merk) | - | - | - | 0.1% |
| pH | 5.6 | 5.6 | 5.6 | 5.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Stelmach-Wityk, K.; Szymonik, K.; Kadluczka, D.; Jedrzejczyk, I.; Grzebelus, E. Effect of Suberoylanilide Hydroxamic Acid and Phytosulfokine-Alpha on Successful Plant Regeneration from Embryogenic Callus-Derived Protoplasts of Garlic (Allium sativum L.). Int. J. Mol. Sci. 2026, 27, 254. https://doi.org/10.3390/ijms27010254
Stelmach-Wityk K, Szymonik K, Kadluczka D, Jedrzejczyk I, Grzebelus E. Effect of Suberoylanilide Hydroxamic Acid and Phytosulfokine-Alpha on Successful Plant Regeneration from Embryogenic Callus-Derived Protoplasts of Garlic (Allium sativum L.). International Journal of Molecular Sciences. 2026; 27(1):254. https://doi.org/10.3390/ijms27010254
Chicago/Turabian StyleStelmach-Wityk, Katarzyna, Kamil Szymonik, Dariusz Kadluczka, Iwona Jedrzejczyk, and Ewa Grzebelus. 2026. "Effect of Suberoylanilide Hydroxamic Acid and Phytosulfokine-Alpha on Successful Plant Regeneration from Embryogenic Callus-Derived Protoplasts of Garlic (Allium sativum L.)" International Journal of Molecular Sciences 27, no. 1: 254. https://doi.org/10.3390/ijms27010254
APA StyleStelmach-Wityk, K., Szymonik, K., Kadluczka, D., Jedrzejczyk, I., & Grzebelus, E. (2026). Effect of Suberoylanilide Hydroxamic Acid and Phytosulfokine-Alpha on Successful Plant Regeneration from Embryogenic Callus-Derived Protoplasts of Garlic (Allium sativum L.). International Journal of Molecular Sciences, 27(1), 254. https://doi.org/10.3390/ijms27010254









