1. Introduction
As global life expectancy increases, aging presents significant challenges to human health and places a growing burden on healthcare systems. Among all organs, the skin, being the largest and most visible, shows the most perceptible signs of aging. Skin aging is a complex process that involves intrinsic and extrinsic mechanisms that lead to various structural and physiological changes [
1]. Common manifestations of skin aging include sagging and wrinkles, primarily due to the loss and degradation of collagen. Collagen, the most abundant protein in mammals, is found extensively in the skin, tendons, bones and other tissues. From early adulthood, collagen levels decline at an approximate rate of 1.5% annually. To counteract this decline and enhance skin collagen, various treatments have been developed, including topical applications such as retinol and tretinoin, as well as advanced technologies like lasers, focused ultrasound and radiofrequencies [
2]. Among these approaches, radiofrequency therapy has gained substantial clinical traction due to its user-friendly application protocol and favorable safety profile.
Radiofrequency technology is a non-invasive treatment that uses high-frequency electrical currents to generate heat energy within the skin tissues. This heat stimulates collagen regeneration and growth, thereby enhancing skin quality and appearance. Additionally, radiofrequency technology promotes improved blood circulation in the skin, contributing to increased radiance and elasticity. Clinically, several studies have demonstrated the efficacy of radiofrequency devices in reducing facial and body skin wrinkles [
3,
4,
5]. In vivo research has confirmed their ability to enhance the density and structural integrity of collagen and elastic fibers. At the molecular level, radiofrequency-induced collagen production has been linked to elevated mRNA levels of transforming growth factor β (TGF-β) and vascular endothelial growth factor (VEGF). Moreover, radiofrequency energy has been shown to boost collagen and elastin synthesis through the up-regulation of NRF2/GLO-1 and modulation of M1/M2 macrophage polarization. It also inhibits the formation of advanced glycation end products (AGEs) and their receptor (RAGE), thereby reducing NF-κB activation and slowing collagen and elastin degradation [
6]. Despite these insights, there remains a gap in understanding of the transcriptional mechanisms and the impact on the tissue microenvironment associated with radiofrequency treatment. For example, what are the biomarkers for the efficacy or sensitivity of radiofrequency treatment? What is the cellular crosstalk in the skin environment during the treatment? To address this, the present study employed RNA sequencing in mouse models to explore additional mechanisms underlying radiofrequencies’ effects on skin collagen production.
3. Discussion
Radiofrequency technology is widely employed in medical aesthetics due to its anti-aging effects, which include reducing skin sagging and wrinkles while enhancing skin firmness, elasticity and radiance. Previous studies have shown that thermal damage caused by radiofrequency treatment initiates the skin’s wound repair process and stimulates collagen regeneration [
14]. However, the molecular mechanisms and gene expression profiles underlying the anti-aging effects of radiofrequencies remain poorly understood. In this study, we observed significant differences in both histological and molecular characteristics of mouse skin following one week of radiofrequency treatment.
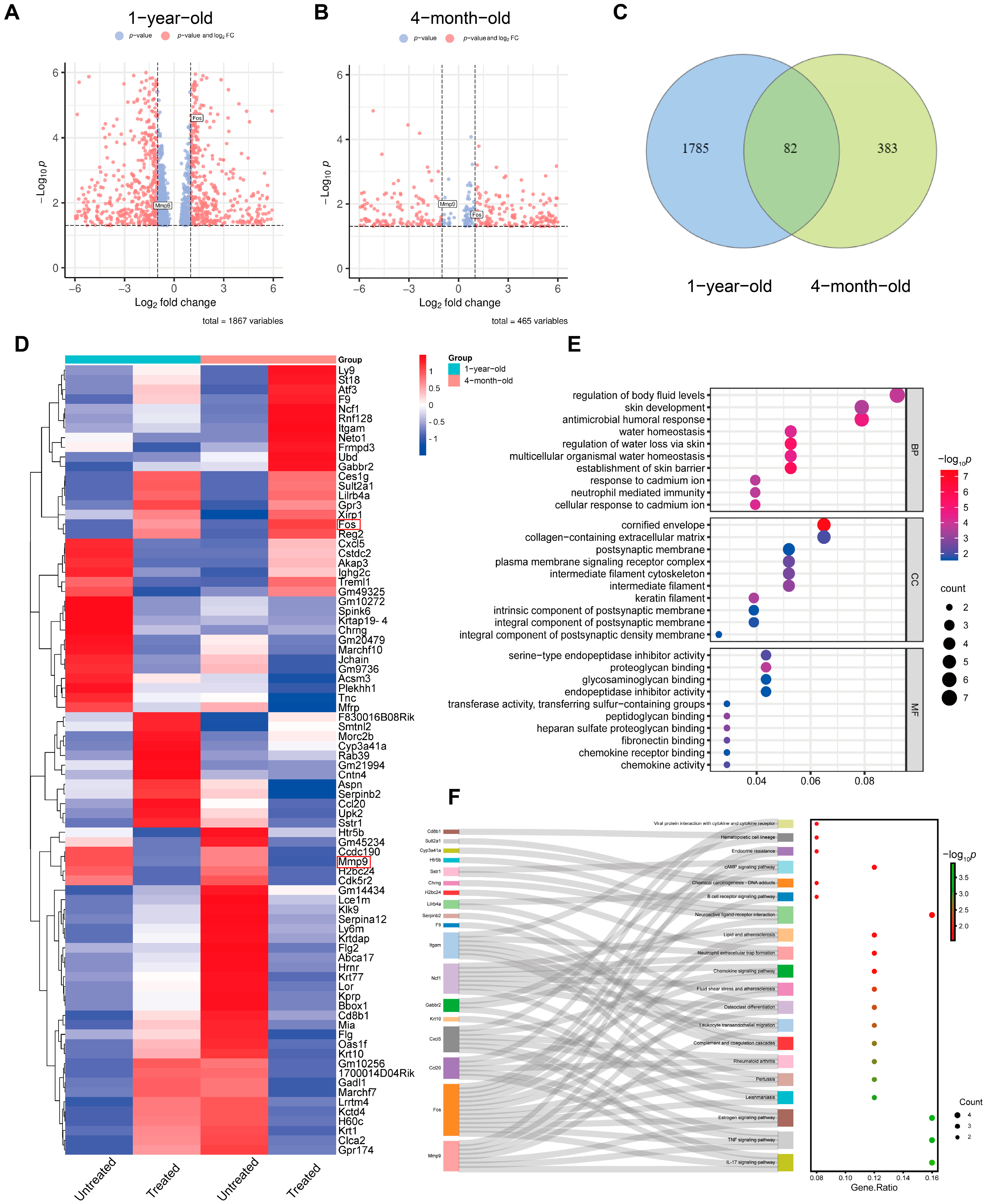
RNA-sequencing analysis of radiofrequency-treated versus untreated mouse skin provides a comprehensive list of significantly differentially expressed genes in the skin. Given the high similarities in histological, physiological and immunological properties between mouse and human skin, the molecular changes observed in our experimental animals are likely to reflect those in human skin treated with radiofrequencies in clinical settings. Besides histological alterations, mouse skin responds to the microthermal damage induced by radiofrequencies with distinct gene expression profiles. A limited number of overlapping differentially expressed genes in the 4-month-old and 1-year-old mice reveal unique gene expression patterns induced by radiofrequencies. These genes include damage response genes such as
Tnc,
Itgam and
Smtnl2.
Tnc and
Itgam are directly involved in damage response, with
Tnc focusing on tissue remodeling and inflammation and
Itgam on immune cell function and response to injury.
Smtnl2, while not as directly linked to damage response as the other two, still plays a role in the physiological processes that can be part of the body’s response to damage, particularly in smooth muscle tissues. We also screened out other genes, which include genes that regulate the cell cycle, DNA replication, cell survival and cytoskeletal remodeling. These genes are
Ly9,
Ncf1,
Fos,
Aspn,
Reg2,
Treml1,
Ccl20,
Chrng,
Atf3,
Marchf7,
Cstdc2,
Lce1m,
Cxcl5,
Akap3,
Acsm3 and
F9. Many of these genes are also up-regulated during wound healing, suggesting that they represent a common transcriptional program involved in cellular stress and proliferation. However, these genes have not been shown to be associated with injury responses. Notably, GO and KEGG analyses highlighted the AP1 family transcription factor Fos. Previous studies demonstrated that mechanical-force-induced skin damage activates the EGFR-RAS-MAPK pathway, leading to Fos up-regulation that drives epidermal stem cell proliferation and renewal [
15]. In our study, we observed consistent Fos up-regulation, suggesting its conserved role in the tissue remodeling process.
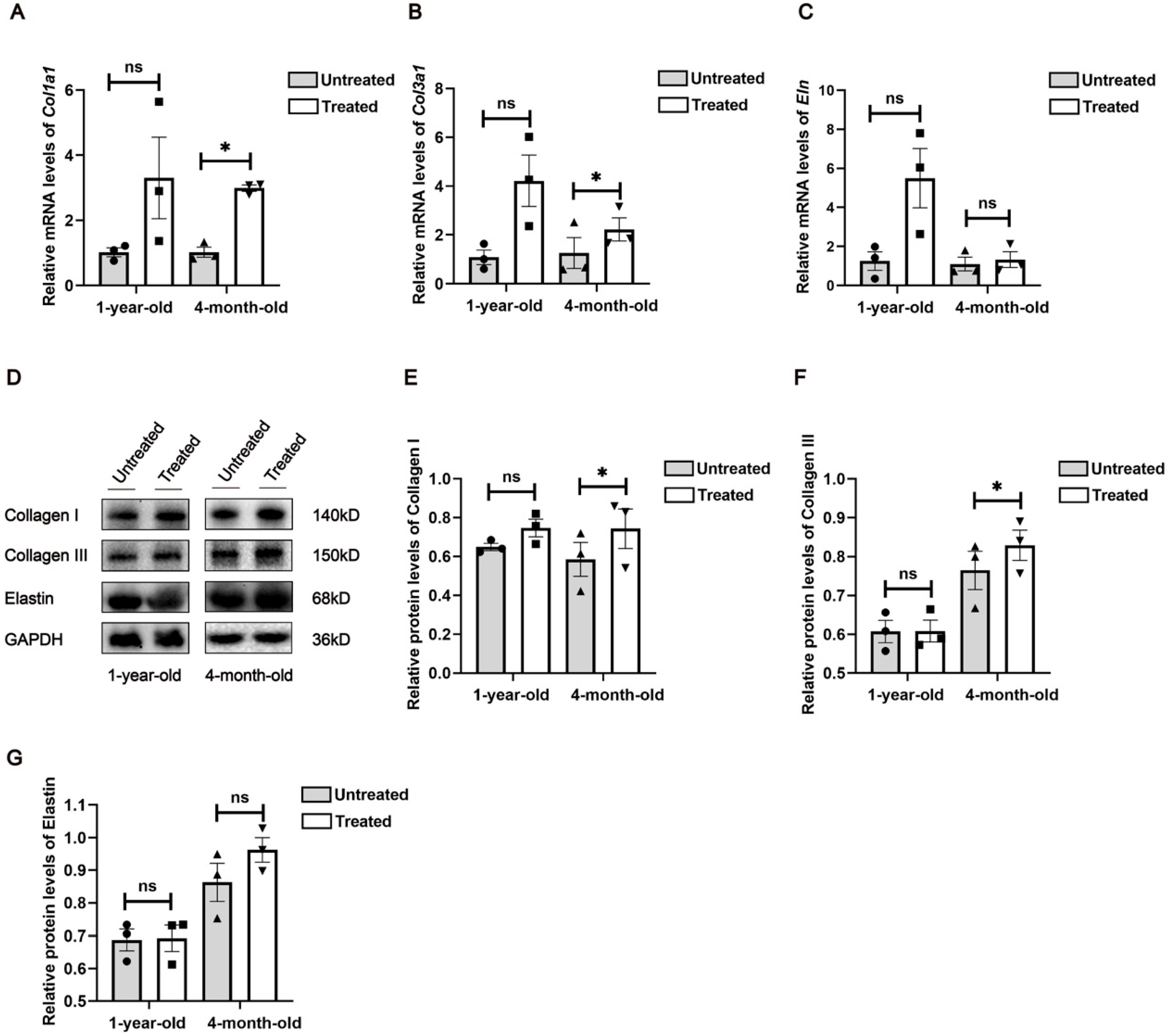
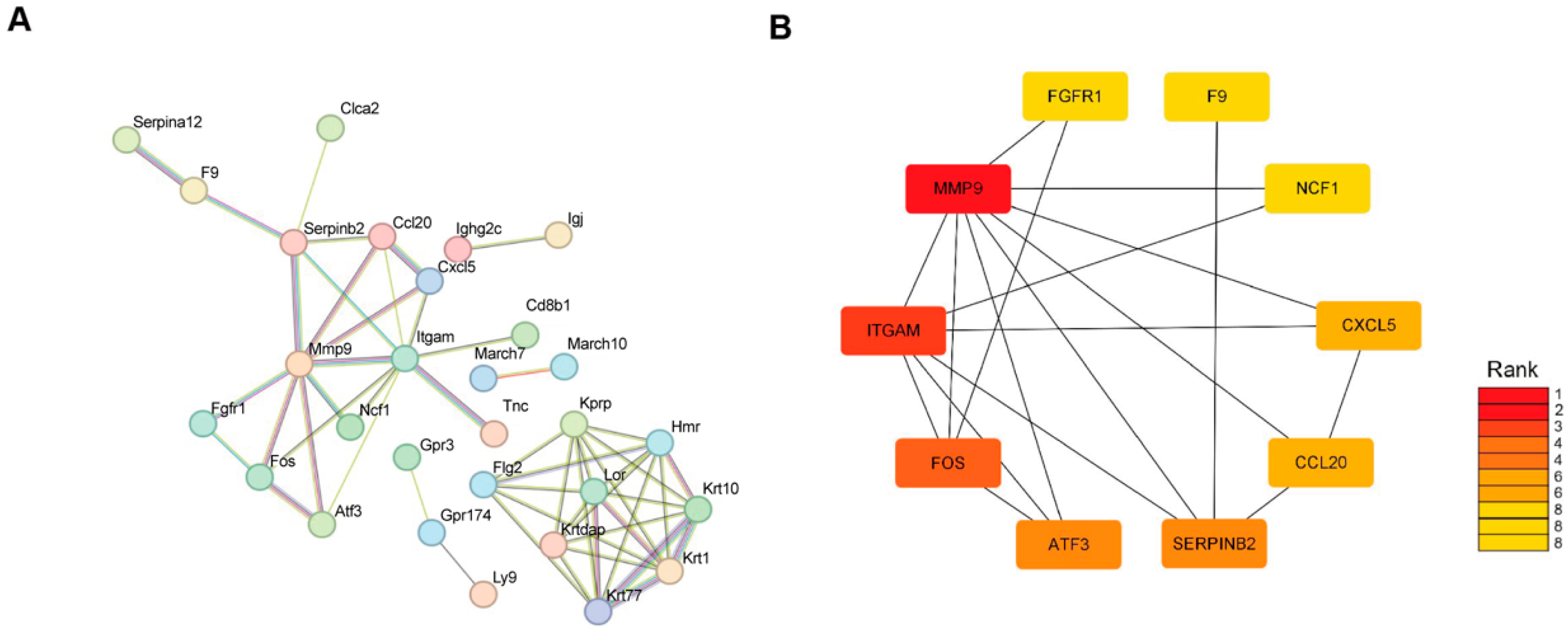
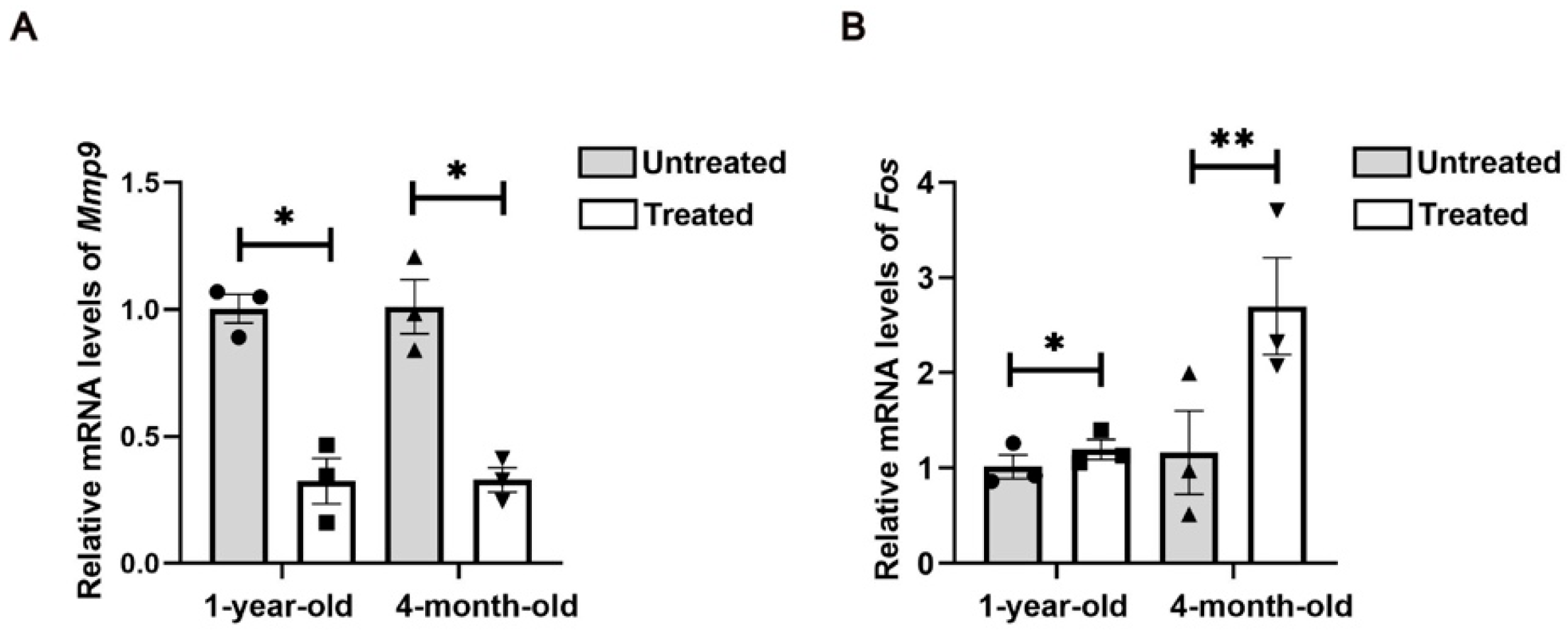
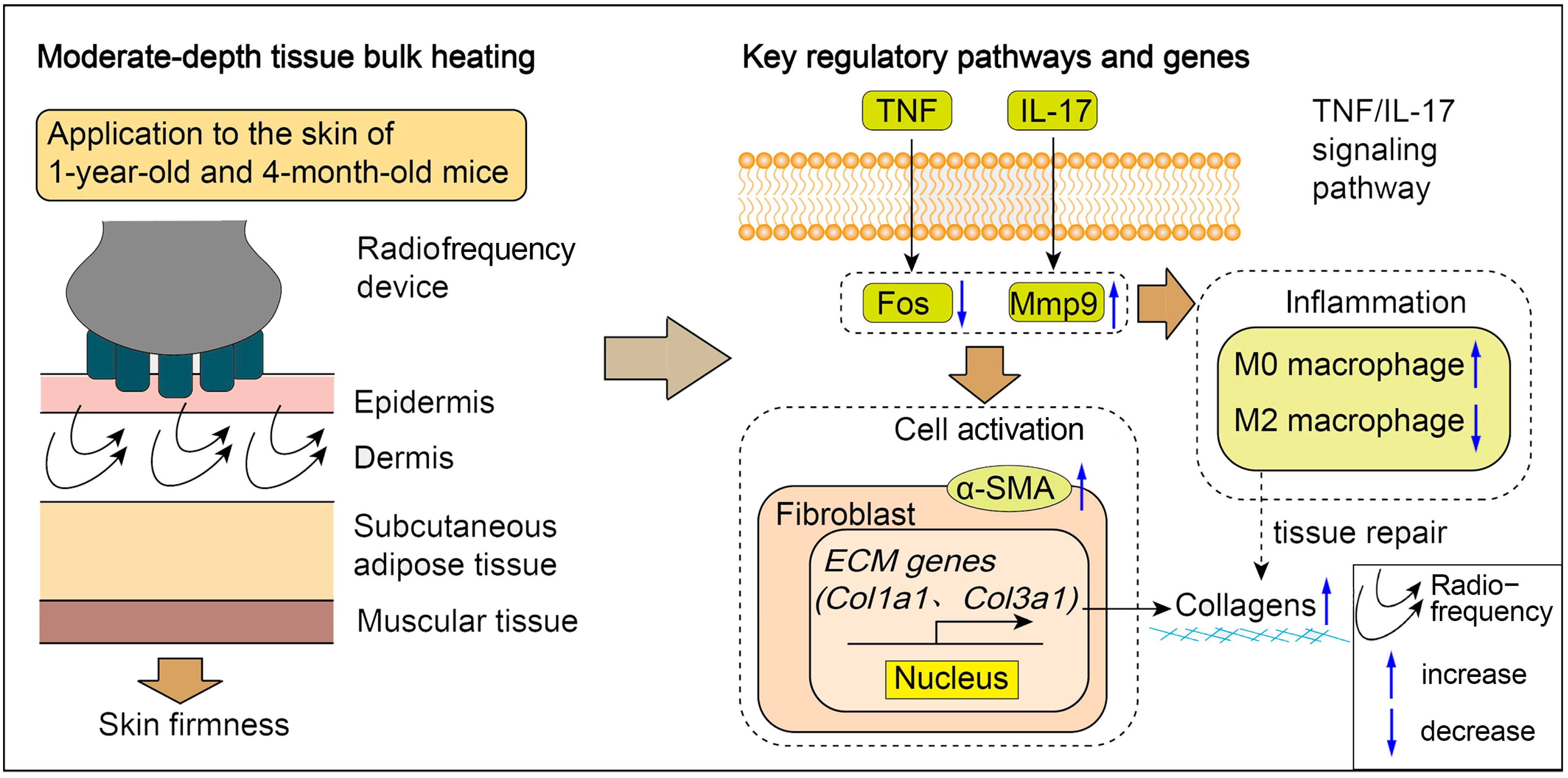
Furthermore, GO and KEGG analyses revealed significant activation of inflammatory responses, including the IL-17 signaling pathway and the TNF signaling pathways, which may stimulate cell proliferation and initiate skin regeneration. Chemokines, cytokines and proteolytic processing factors interact in inflammatory responses induced by microthermal injury. In this complex regulatory network, we observed changes in the activity of MMP genes involved in ECM remodeling following radiofrequency treatment. Mmp9, a matrix metalloproteinase, degrades collagen, gelatin, fibrin and other ECM components [
16]. Differential expression analysis indicates that Mmp9 expression is down-regulated after radiofrequency treatment in both young and aged mice, which suggests a reduction potential in ECM degradation. Our findings underscore the critical involvement of inflammatory cascades in mediating cutaneous adaptive responses to radiofrequency exposure. Future investigation is needed to delineate the mechanisms through which controlled immune activation orchestrates tissue repair post-thermal injury, particularly focusing on the temporal regulation of pro-regenerative versus pro-fibrotic signaling pathways. Furthermore, the therapeutic potential of combinatorial regimens integrating anti-inflammatory immunomodulators with radiofrequency devices warrants systematic exploration in preclinical models.
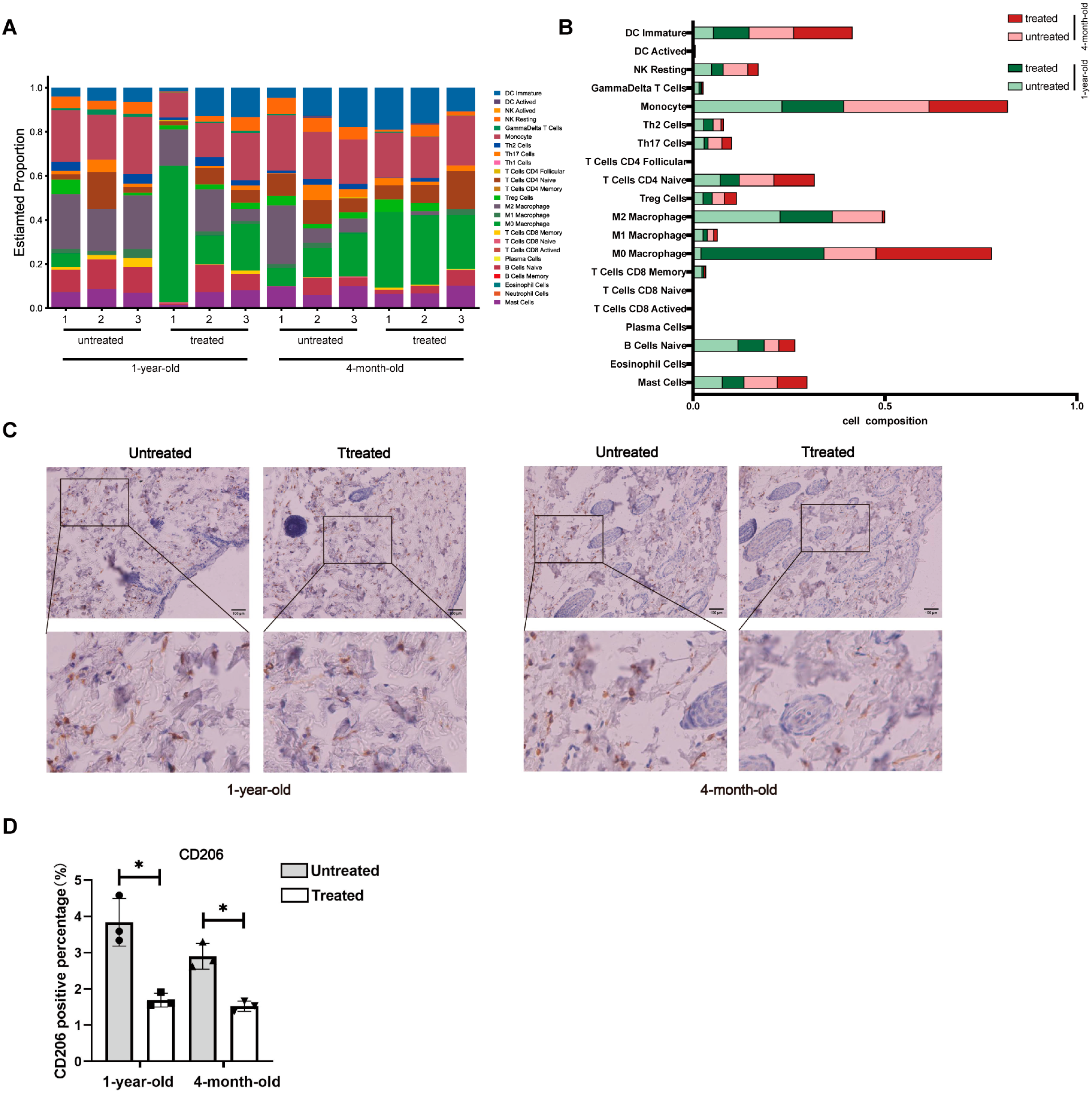
Although histological analysis indicated significant differences between the young and aged groups, as well as between the untreated and the treated groups, the most pronounced molecular differences were observed in collagen-related genes and proteins within the young group (4-month-old mice). The observed discrepancy in radiofrequency efficacy between the 4-month-old group and the 1-year-old group may be attributed to the age-related alterations in the tissue microenvironment that collectively impair regenerative capacity. Mechanistically, aged skin exhibits (1) dysfunctional collagen deposition characterized by a fragmented matrix architecture that disrupts fibroblast adhesion and mechanotransduction signaling, (2) altered immune cell dynamics with increased M2-polarized macrophages that may dampen pro-regenerative crosstalk between fibroblasts and immune compartments [
17], and (3) diminished expression of critical transcriptional regulators (e.g., AP1 factors) and chemokine gradients essential for orchestrating biomolecular cascades following thermal stimulation [
18]. These degenerative changes are further exacerbated by age-associated adipose infiltration that physically disrupts dermal architecture [
19]. Consequently, these alterations create a microenvironment less responsive to radiofrequency-induced tissue remodeling signals compared to younger skin with intact ECM integrity and robust paracrine signaling networks.
There are also limitations to our study. Although we identified Fos and MMP9 as the targets of radiofrequencies in skin aging treatment, validation of these targets using either genetic or pharmacologic manipulation has not yet been fulfilled. The age-related radiofrequency resistance due to alterations in the microenvironment still needs to be investigated in cohorts with large sample sizes and long-term observations, as well as radiofrequencies with more parameters. The detailed crosstalk among cells in skin ECM during tissue repair is worth exploring.
5. Materials and Methods
5.1. Mice and Radiofrequency Treatment
ICR male mice, aged 6–8 weeks, were obtained from Shanghai SLAC Animal Co. (Shanghai, China) and were housed in the animal facility at the Nanjing University of Chinese Medicine, with a temperature of 24 °C as well as a 12 h/12 h light/dark cycle, with ad libitum access to food and water until 4 months of age and 1 year of age. The animals were monitored and cared for according to the guidelines set by the Animal Ethics Committee of Nanjing University of Chinese Medicine (approval number: 202209A069). The mice were randomly divided into two age groups upon their arrival at the animal facility: 4-month-old mice (n = 6) and 1-year-old mice (n = 5).
The animal experiments were designed as self-control studies in two age groups, where each subject served as its own control. This approach allowed for a direct comparison of effects within the same individual, thereby reducing variability and enhancing the reliability of the results. Prior to each treatment, the mice were anesthetized using isoflurane inhalation. Mouse dorsal skin was delineated into two 1 cm × 2 cm areas: one designated as untreated and the other as treated, following hair removal. The dorsal skin was cleaned and dried thoroughly. A gel (TriPollar
®, Pollogen Ltd., Tel Aviv, Israel) was evenly applied to the designated treatment area. A multipolar radiofrequency device (model: TriPollar
® Stop VX2, Pollogen Ltd.) was utilized in low-range mode, delivering a power density of 8 W/cm
2, with frequencies alternating between 0.9, 1.0 and 1.25 MHz. The dorsal skin was massaged until the integrated temperature indicator activated (target range: 40–42 °C), ensuring consistent application across the treated area. Treatments were conducted every other day for one week (a regimen optimized based on prior unpublished data showing peak mRNA profiles of collagen type I and type III and fibroblast activity at this interval;
Figure 1A). Following the final treatment, the mice were euthanized via isoflurane inhalation (4% in oxygen) and cervical dislocation, and dorsal skin tissues were harvested for analysis.
5.2. Histopathological Analysis
Dorsal skin samples were fixed in 4% paraformaldehyde for 24 h, followed by embedding in paraffin and microtome sectioning. Sections were prepared with a 4 μm thickness and were stained with hematoxylin and eosin (H&E). To assess collagen fiber content, additional sections were stained with Masson’s trichrome. The stained sections were then imaged using the Mantra Pathology Workstation (PerkinElmer, Waltham, MA, USA). Dermal thickness and collagen fiber volume were quantified using ImageJ software (v1.8.0.112, Bethesda, MD, USA), based on measurements from the H&E and Masson’s trichrome staining, respectively.
5.3. Immunohistochemical (IHC) Staining
IHC staining was conducted following the manufacturer’s protocol (ZSGB-BIO, Beijing, China). Briefly, tissue sections were incubated overnight at 4 °C with the primary antibody CD206 (CST, 24595T, Danvers, MA, USA). This was followed by a 50 min incubation at room temperature with HRP-labeled Goat Anti-Rabbit IgG (H+L) (Bioworld, BS13278, Nanjing, China). Antigen–antibody complexes were visualized using diaminobenzidine (DAB). The stained sections were imaged using the Mantra Pathology Workstation (PerkinElmer, Waltham, MA, USA).
5.4. Immunofluorescence (IF) Staining
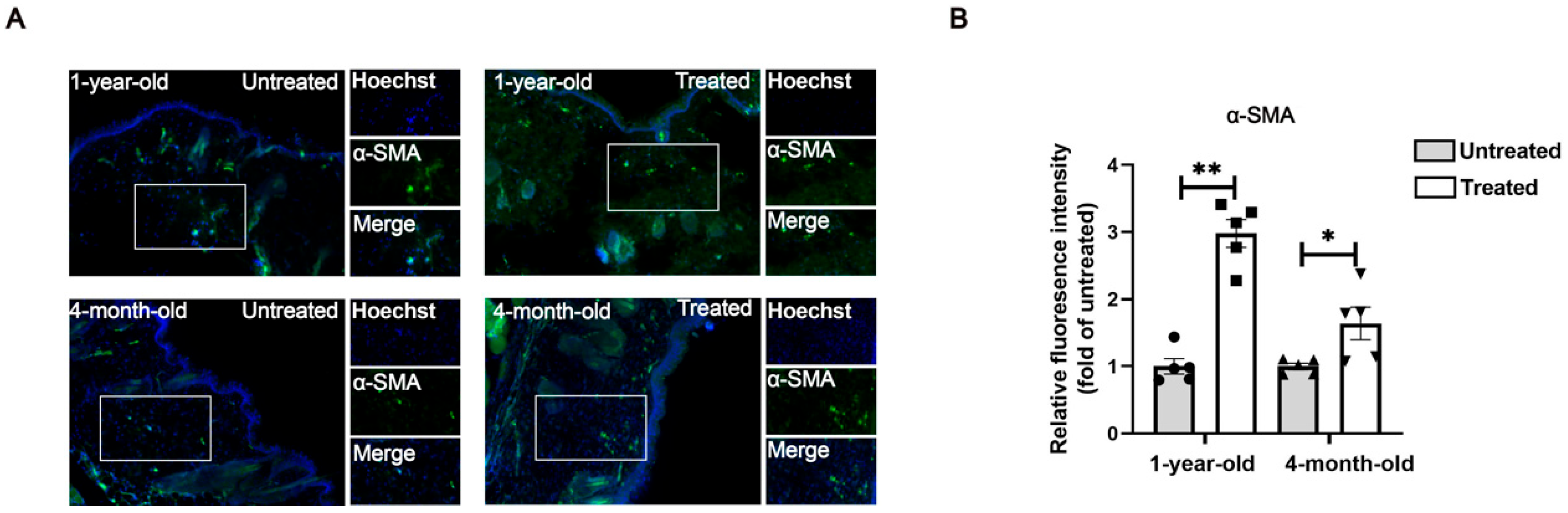
For tissue immunofluorescence staining, paraffin-embedded skin sections fixed with 4% paraformaldehyde (PFA) were deparaffinized and dehydrated through a series of xylene and ethanol washes. Antigen retrieval was performed in sodium citrate buffer. After blocking with 5% BSA for 30 min at room temperature, sections were incubated overnight at 4 °C with the primary antibody α-SMA (CST, 19245T, Danvers, MA, USA). Following this, the sections were incubated with the secondary antibodies for 1 h at room temperature. Sections were counterstained with Hoechst33342 to label nuclei. Images were captured from three random fields for each sample using a Zeiss Axiovert A1 microscope (Oberkochen, Germany).
5.5. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
Dorsal skin tissues were snap-frozen with liquid nitrogen and homogenized with a micro tissue homogenizer (Servicebio, KIZ-III-F, Wuhan, China). Total RNA was isolated using TRIzol (Invitrogen/Life Technologies, Carlsbad, CA, USA) following the manufacturer’s protocol. The extracted RNA was quantified by NanoDrop (Thermo Fisher Scientific, ND-ONE-W, Waltham, MA, USA) and converted to cDNA using a reverse transcription kit (Vazyme, R223, Nanjing, China). The primers used are listed in
Table 1, and qPCR was performed on an Applied Biosystems 7900HT (Applied Biosystems/Life Technologies, Foster City, CA, USA). Glyceraldehyde-3-phosphate dehydrogenase (Gaphd)-normalized mRNA for specific gene expression was calculated by the 2
−ΔΔCt method.
5.6. Western Blot Analysis
Protein samples from mouse dorsal skin were homogenized using a micro tissue homogenizer and then lysed with RIPA buffer, following the manufacturer’s instructions (Yeasen, 20115ES60, Shanghai, China). Protein concentration was determined with a BCA protein assay kit (Beyotime, P0012, Shanghai, China) according to the manufacturer’s instructions. For Western blotting, 30 μg of protein per sample was loaded on a sodium dodecyl sulfate–polyacrylamide (SDS) gel and separated by electrophoresis. The proteins were then transferred to polyvinylidene fluoride (PVDF) membranes using the wet transfer system (Bio-Rad, MiniProtean 3 Cell, San Francisco, CA, USA). The PVDF membranes were blocked with 5% non-fat milk in Tris-buffered saline and Tween 20 (TBST) and then incubated overnight at 4 °C with primary antibodies against type I collagen (Proteintech, 66761-1-Ig, Wuhan, China), type III collagen (Proteintech, 22734-1-AP, Wuhan, China) and elastin (Immunoway, YN2891, Suzhou, China), diluted in TBST containing 0.02% sodium azide and 5% BSA. After washing 4 times with TBST, the membrane was incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Bioworld Technology, BS13278, BS12478, Nanjing, China). The protein blot was visualized using enhanced chemiluminescence (ECL) reagents (Biosharp, BL520B, Hefei, China) and captured with a Bio-Rad imaging system (Bio-Rad, XRS+, San Francisco, CA, USA).
5.7. Image Analysis
The pathological tissue images and Western blot images were analyzed using ImageJ software in a blind manner. Specifically, all images were randomized and labeled by an independent researcher prior to analysis, ensuring that the analyst was unaware of the treatment groups. This approach minimized bias during the quantification of relevant parameters, allowing for objective evaluation of the histological features. Image analysis was performed with ImageJ software (v1.8.0.112, Bethesda, MD, USA).
5.8. RNA Sequencing
Sample selection and data exclusion: Samples were selected based on pathological phenotype and RNA stability, with three biological replicates per group included in the analysis. The samples from the same three biological replicates per group were subsequently used for molecular experiments (Western blot and qPCR analyses) to ensure consistency. No additional samples or data points were excluded beyond this selection criteria.
Tissue collection: Mouse skin tissues (about 100 mg per biological replicate) were harvested from each experimental group, immediately snap-frozen in liquid nitrogen and stored at −80 °C until processing.
RNA extraction: Total RNA from untreated and treated skin tissues was extracted using Trizol (Invitrogen, Carlsbad, CA, USA), following the manufacturer’s protocol, with 3 replicates per group according to the manufacturer’s instructions. RNA quantity and integrity was measured using the Nanodrop One (Thermo Fisher Scientific, MA, USA) and the Agilent 2100 Bioanalyzer (Agilent Technologies, PaloAlto, CA, USA), respectively. RNA degradation was examined by RNase-free agarose gel electrophoresis.
RNA sequencing: The mRNA was enriched by oligo(dT) magnetic beads, cut into short fragments and reverse transcribed into the first-strand cDNA using the First Strand Synthesis Reaction Buffer and Random Primer Mix (2×). The second-strand cDNAs were synthesized using the Second Strand Synthesis Reaction Buffer (10×) and Second Strand Synthesis Enzyme Mix. The library was constructed using the NEB Next Ultra RNA Library Prep Kit for Illumina (neb# 7530, New England Biolabs, Ipswich, MA, USA). Subsequently, the second-strand cDNAs were purified with AMPure XP Beads. The ends were repaired, poly(A) was added and the cDNA was ligated to Illumina sequencing adapters. The ligation products were purified using AMPure XP Beads (1.0×X), washed with 80% ethanol and eluted with ddH2O. The connecting products were used for PCR amplification. The cDNA library was constructed and sequenced on the Illumina sequencing platform (Illumina Novaseq6000) supplied by Gene Denovo Biotechnology Co. (Guangzhou, China).
Gene expression levels were normalized by the FPKM (fragments per thousand base transcripts/million map readouts) method, and the differential expression genes were screened with a fold change of ≥1.2 and a
p-value < 0.05. Volcano maps and heat maps were generated using online website tools (
https://www.omicsmart.com/), and GO enrichment and KEGG pathway analyses were generated using the clusterProfiler R package (v4.0) with a significance threshold of
p < 0.05. Abundances of immune cell infiltration were analyzed by the Cibersort algorithm. Two-way ANOVA analysis was used to assess the differences in the proportions of immune cells. Results with
p < 0.05 were considered significant. The RNA-sequencing data are available in the Gene Expression Omnibus (GEO) database under GSE278079.
5.9. Statistical Analysis
The paired Student’s t-test was used to assess significance for all data sets. GraphPad 8.02 software (version 8.02; San Diego, CA, USA) analyzed and processed the data results. Quantitative analysis of histological experiments was performed with either five or six biological replicates, as indicated individually. Data sets were analyzed with three biological replicates in the Western blot and qPCR experiments. Data were expressed as means ± standard deviations. p < 0.05 indicated a statistically significant difference between groups.